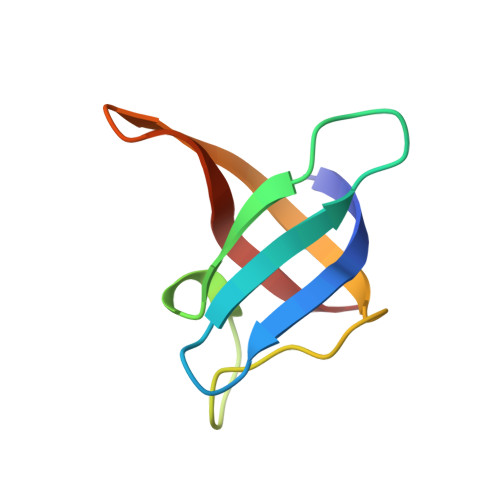
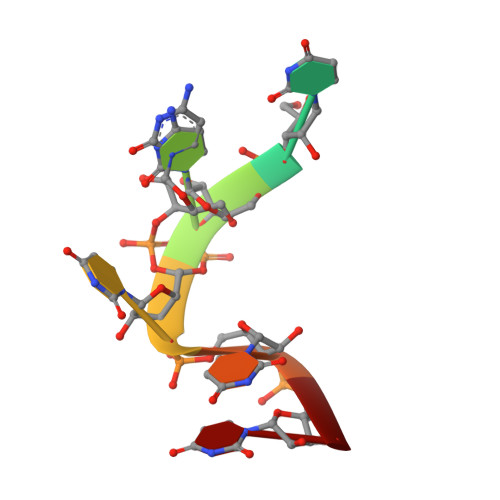
RNA single strands bind to a conserved surface of the major cold shock protein in crystals and solution.
Sachs, R., Max, K.E., Heinemann, U., Balbach, J.(2012) RNA 18: 65-76
- PubMed: 22128343
- DOI: https://doi.org/10.1261/rna.02809212
- Primary Citation of Related Structures:
3PF4, 3PF5 - PubMed Abstract:
Bacterial cold shock proteins (CSPs) regulate the cellular response to temperature downshift. Their general principle of function involves RNA chaperoning and transcriptional antitermination. Here we present two crystal structures of cold shock protein B from Bacillus subtilis (Bs-CspB) in complex with either a hexanucleotide (5'-UUUUUU-3') or heptanucleotide (5'-GUCUUUA-3') single-stranded RNA (ssRNA). Hydrogen bonds and stacking interactions between RNA bases and aromatic sidechains characterize individual binding subsites. Additional binding subsites which are not occupied by the ligand in the crystal structure were revealed by NMR spectroscopy in solution on Bs-CspB·RNA complexes. Binding studies demonstrate that Bs-CspB associates with ssDNA as well as ssRNA with moderate sequence specificity. Varying affinities of oligonucleotides are reflected mainly in changes of the dissociation rates. The generally lower binding affinity of ssRNA compared to its ssDNA analog is attributed solely to the substitution of thymine by uracil bases in RNA.
- Fachgruppe Biophysik Institut für Physik, Martin-Luther-Universität Halle-Wittenberg, 06120 Halle (Saale), Germany.
Organizational Affiliation: