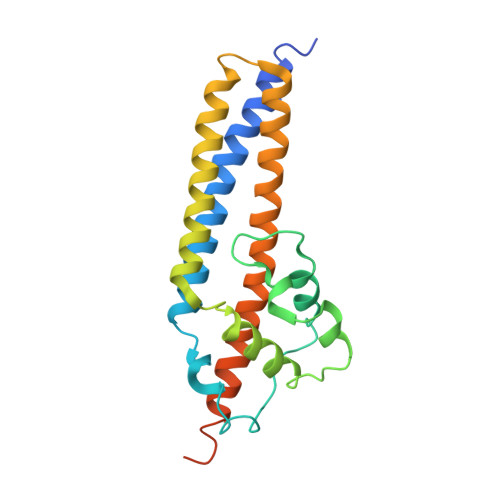
Structural and functional characterization of the VirB5 protein from the type IV secretion system encoded by the conjugative plasmid pKM101
Yeo, H.-J., Yuan, Q., Beck, M.R., Baron, C., Waksman, G.(2003) Proc Natl Acad Sci U S A 100: 15947-15952
- PubMed: 14673074
- DOI: https://doi.org/10.1073/pnas.2535211100
- Primary Citation of Related Structures:
1R8I - PubMed Abstract:
Type IV secretion systems mediate intercellular transfer of macro-molecules via a mechanism ancestrally related to that of bacterial conjugation machineries. TraC of the IncN plasmid pKM101 belongs to the VirB5 family of proteins, an essential component of most type IV secretion systems. Here, we present the structure of TraC. VirB5/TraC is a single domain protein, which consists of a three helix bundle and a loose globular appendage. Structure-based site-directed mutagenesis followed by functional studies indicates that VirB5 proteins participate in protein-protein interactions important for pilus assembly and function.
Organizational Affiliation:
Institute of Structural Molecular Biology, Birkbeck College, Malet Street, London WC1E 7HX, United Kingdom.