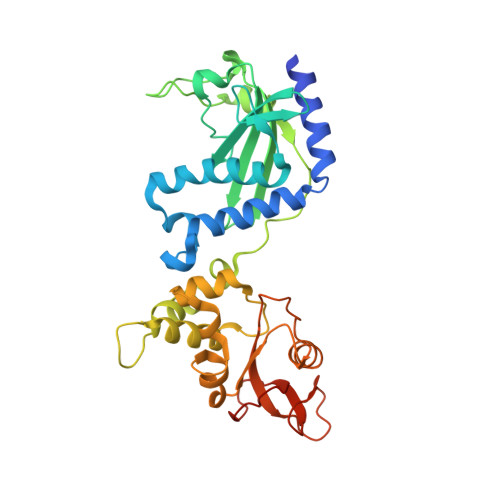
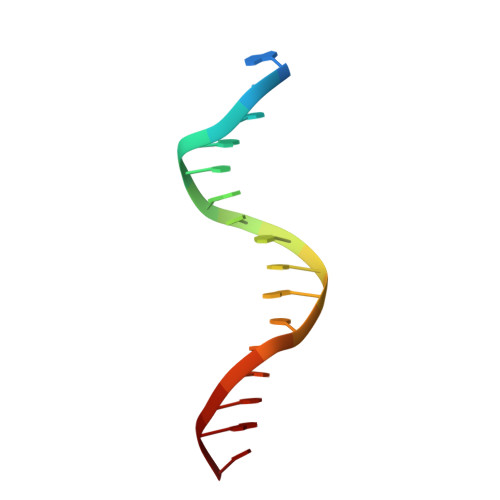
Structure of NaeI-DNA complex reveals dual-mode DNA recognition and complete dimer rearrangement.
Huai, Q., Colandene, J.D., Topal, M.D., Ke, H.(2001) Nat Struct Biol 8: 665-669
- PubMed: 11473254
- DOI: https://doi.org/10.1038/90366
- Primary Citation of Related Structures:
1IAW - PubMed Abstract:
NaeI, a novel DNA endonuclease, shows topoisomerase and recombinase activities when a Lys residue is substituted for Leu 43. The NaeI-DNA structure demonstrates that each of the two domains of NaeI recognizes one molecule of DNA duplex. DNA recognition induces dramatic rearrangements: narrowing the binding site of the Topo domain 16 A to grip DNA, widening that of the Endo domain 8 A to encircle and bend DNA 45 degrees for cleavage, and completely rebuilding the homodimer interface. The NaeI-DNA structure presents the first example of novel recognition of two copies of one DNA sequence by two different amino acid sequences and two different structural motifs in one polypeptide.
Organizational Affiliation:
Department of Biochemistry and Biophysics, The University of North Carolina, Chapel Hill, North Carolina 27599-7260, USA.