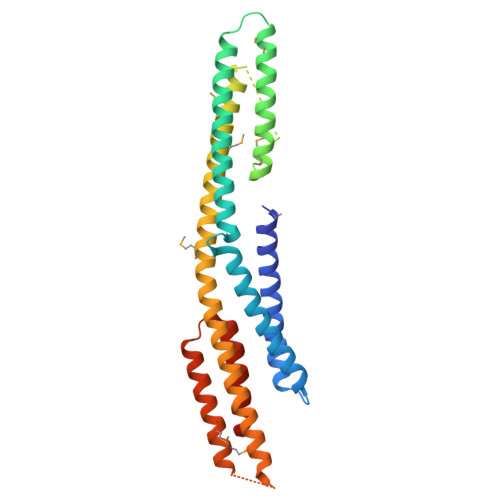
Atg23 Interacts With Both the N- and C-termini of Atg9 Via a Hydrophobic Binding Pocket.
Bekkhozhin, Z., Leary, K.A., Ragusa, M.J.(2025) bioRxiv
- PubMed: 41446224
- DOI: https://doi.org/10.64898/2025.12.17.694986
- Primary Citation of Related Structures:
9ZM0 - PubMed Abstract:
Macroautophagy is a cellular process where cytosolic material is captured in double membrane vesicles, termed autophagosomes, which fuse with the vacuole or lysosomes leading to the degradation of the captured contents. In yeast, the biogenesis of autophagosomes is initiated by the fusion of a few small vesicles which contain the integral membrane protein Atg9. Atg9 vesicle trafficking is in part regulated by the peripheral membrane protein Atg23. However, the structure of Atg23 and the mechanism by which Atg23 interacts with Atg9 are currently unknown. Therefore, we determined the crystal structure for a monomeric form of Atg23 and characterized the interaction between Atg23 and Atg9. This work reveals that Atg23 contains a novel fold which is consistent with the AlphaFold 3 prediction except that the helices running towards the dimerization region have a bend giving a more curved global architecture than the prediction. In addition, we demonstrate that conserved sequences in both the N and C-terminal regions of Atg9 bind to a hydrophobic cavity on Atg23.
- Department of Chemistry, Dartmouth College, Hanover, NH 03755, USA.
Organizational Affiliation: