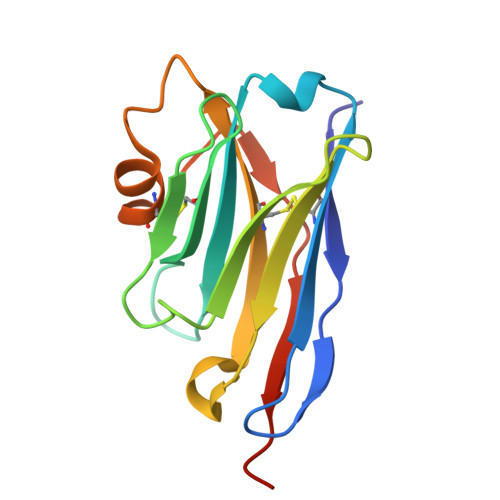
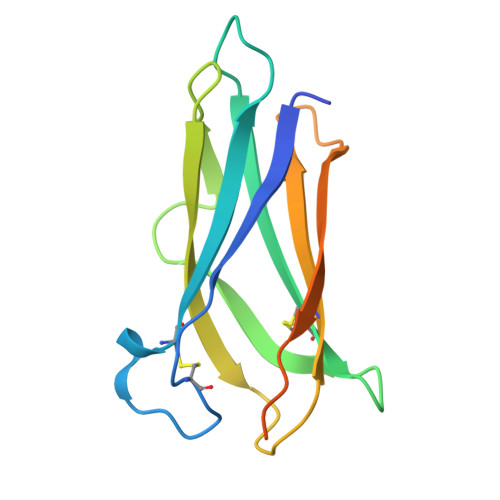
Crystal structure and nanobodies against domain 3 of the malaria parasite fusogen Plasmodium falciparum HAP2.
Lyons, F.M.T., Chmielewski, J., Chan, L.J., Gabriela, M., Chan, R.W.B., Adair, A., Tong, J., Pymm, P., Zeglinski, K., Gouil, Q., Tham, W.H., Dietrich, M.H.(2026) Biochem J 483
- PubMed: 41498187
- DOI: https://doi.org/10.1042/BCJ20250297
- Primary Citation of Related Structures:
9YC3 - PubMed Abstract:
Malaria parasites are transmitted to humans through a bite from an infected female Anopheles mosquito. Within the mosquito midgut, malaria parasite gametes are activated and undergo fertilisation. If parasite fertilisation is perturbed, this stops the transmission of malaria parasites from mosquito to human. One proposed target of transmission-blocking interventions is Plasmodium falciparum fusogen PfHAP2, which is essential for gamete fusion during parasite fertilisation. However, to date, no monoclonal antibodies or structures of PfHAP2 have been generated. We have identified nanobodies that bind specifically to domain 3 of PfHAP2 with nanomolar affinities, two of which show some cross-species reactivity with HAP2 of other Plasmodium species. The crystal structure of one nanobody in complex with domain 3 of PfHAP2 provides the first structural insights into this transmission-blocking target in P. falciparum.
- Walter and Eliza Hall Institute of Medical Research, Parkville, Australia.
Organizational Affiliation: