Design, synthesis, and structural analysis of an inhibitor of the gastric proton pump with a diaza-tricyclic skeleton.
Umekubo, N., Hashizume, A., Saito, H., Kato, S., Kanai, C., Gopalasingam, C.C., Gerle, C., Shigematsu, H., Yoshimori, A., Abe, K., Yokoshima, S.(2026) Org Biomol Chem
- PubMed: 41536160
- DOI: https://doi.org/10.1039/d5ob01828e
- Primary Citation of Related Structures:
9VVO - PubMed Abstract:
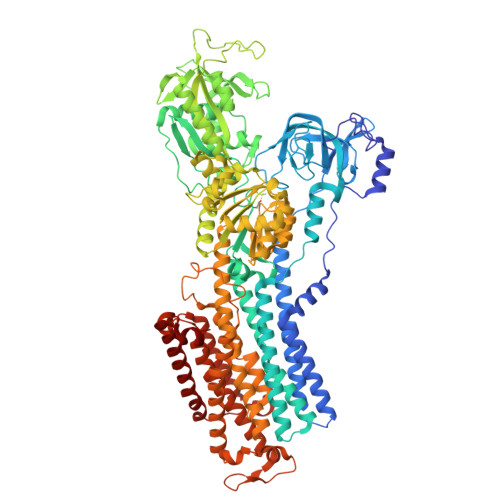
The development of potent K + -competitive acid blockers (P-CABs) as inhibitors of acid gastric secretion attracts much research attention. In this study, the structure-guided design and enantioselective synthesis of P-CABs yielded a diaza-tricyclic compound with moderate inhibitory activity against the gastric proton pump. The eutomer was experimentally confirmed, consistent with pharmacophore predictions, and its binding mode to the gastric proton pump was elucidated via cryo-electron microscopy.
- Graduate School of Pharmaceutical Sciences, Nagoya University, Nagoya, Aichi 464-8601, Japan. yokoshima.satoshi.v7@f.mail.nagoya-u.ac.jp.
Organizational Affiliation: