Human Proteoglycan Linkage Region Glycosyltransferases are Dimeric and Show Unexpected Specificities.
Weidler, S., Bundgaard, O., Hessefort, M., Radisch, M., Graf, C.G.F., Lam, K., Neubauer, V.J., Eisenreich, J., Kohler, L., Moremen, K.W., Steentoft, C., Clausen, H., Huang, T.Y., Hung, S.C., Steegborn, C., Weyand, M., Unverzagt, C.(2026) Angew Chem Int Ed Engl 65: e16855-e16855
- PubMed: 41316887
- DOI: https://doi.org/10.1002/anie.202516855
- Primary Citation of Related Structures:
9SP7 - PubMed Abstract:
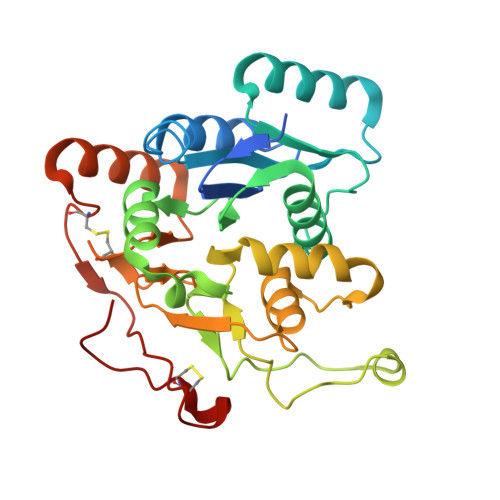
We selected the N,O-glycosylated proteoglycan bikunin as a model to establish a chemoenzymatic approach to defined proteoglycans using native chemical ligation. Overexpression of the human linkage region glycosyltransferases B4GalT7, B3GalT6 and B3GlcAT-1 as N-terminal SUMO-fusions gave high yields of soluble and active enzymes in E. coli. When starting with xylosylated bikunin peptides the transferases performed well in enzymatic cascade reactions and provided the desired linkage region tetrasaccharide glycopeptides. B3GalT6 and B3GlcAT-1 led to side products with N,O-glycosylated bikunin peptides revealing unexpected promiscuity of both enzymes towards complex type N-glycans. Additionally, B3GalT6 was found to synthesize short poly-β3 Gal structures. B3GlcAT-1 can slowly convert the biosynthetic intermediate Gal-Xyl to the non-canonical trisaccharide GlcA-Gal-Xyl. This reaction independently confirmed the recently detected biosynthetic bypass to GAGs in the case of dysfunctional B3GalT6 (spondylodysplastic Ehlers-Danlos-syndrome). The three linkage region glycosyltransferases B4GalT7, B3GalT6 and B3GlcAT-1 were dimeric in solution and the crystal structure of B3GalT6 was solved showing a covalent dimer linked by a disulfide in the center of the large dimerization domain. This motif appears to be conserved in higher organisms and reinforces the concept of dimeric glycosyltransferases lining the Golgi.
- University of Bayreuth, Bioorganic Chemistry, Universitätsstraße 30, 95447, Bayreuth, Germany.
Organizational Affiliation: