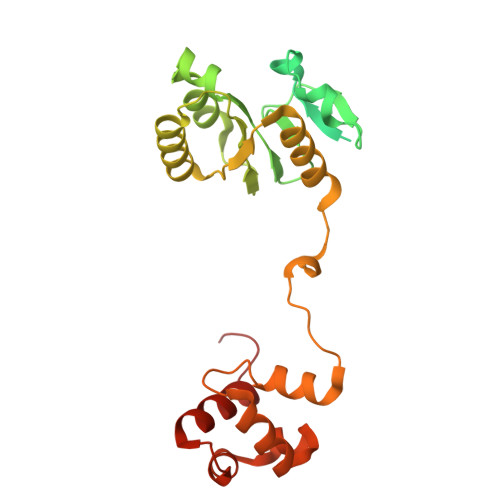
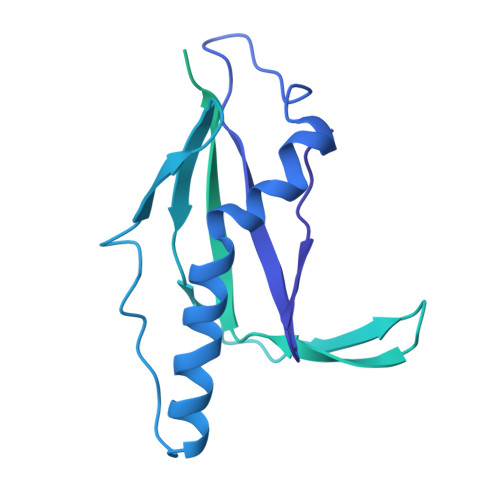
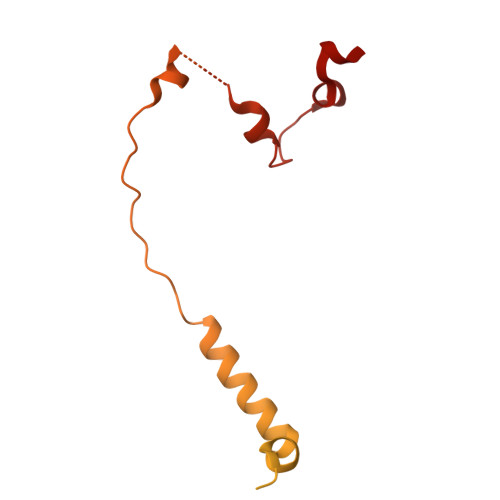
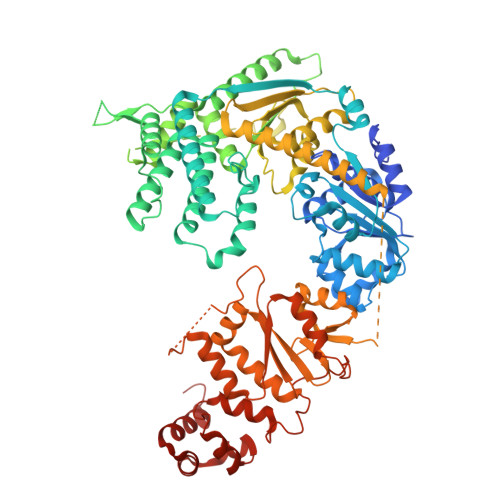
Molecular basis of XPF-ERCC1 targeting to SLX4-dependent DNA repair pathways.
Feng, J., Martin, P.R., Kowalski, S., Lecot, M., Cronin, N.B., Matthews-Palmer, T., Niedzwiedz, W., Greber, B.J.(2025) Nat Commun 17: 522-522
- PubMed: 41402316
- DOI: https://doi.org/10.1038/s41467-025-67216-3
- Primary Citation of Related Structures:
9QEC, 9QED, 9QEE - PubMed Abstract:
The preservation and faithful propagation of genetic information is essential for all life forms and depends on cellular pathways that enable replication, recombination, and repair of DNA. The multifunctional XPF-ERCC1 DNA endonuclease complex acts in several DNA repair pathways and interacts with numerous partner proteins and large DNA repair assemblies, including the nucleotide excision repair machinery and the SMX tri-endonuclease complex. Here, we report structures of XPF-ERCC1 in complex with the DNA repair factors SLX4 and SLX4IP, thereby identifying key residues responsible for direct interactions with XPF-ERCC1. When introduced into human cells, point mutations in these interfaces impair the interactions between XPF-ERCC1 and SLX4 or SLX4IP, and disruption of the XPF-SLX4IP interface leads to cis-platin sensitivity. Furthermore, our data reveal the structure of the human XPF-ERCC1-SLX4IP-SLX4 330-555 complex with DNA bound at its active site, and they complete the structural characterisation of molecular interactions required to assemble the SMX complex.
- Division of Structural Biology, The Institute of Cancer Research, Chester Beatty Laboratories, London, UK.
Organizational Affiliation: