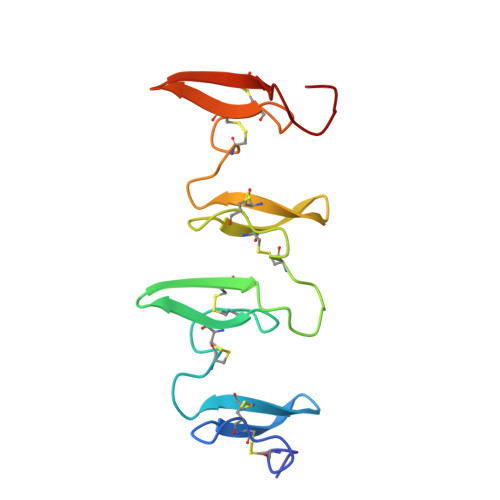
Structure of the disulfide-rich modules of a striking tandem repeat protein, avian cysteine-rich eggshell membrane protein.
Zeinalilathori, S., Russell, R.W., Ross, J.L., Modla, S., Caplan, J.L., Polenova, T., Thorpe, C.(2026) Protein Sci 35: e70431-e70431
- PubMed: 41432289
- DOI: https://doi.org/10.1002/pro.70431
- Primary Citation of Related Structures:
9PGB - PubMed Abstract:
Avian eggshell membrane (ESM) is fabricated within the isthmus region of the oviduct and is comprised of three juxtaposed, predominantly proteinaceous layers lying between egg white and the calcified shell. The limiting membrane is less than 0.5 μm in thickness and forms the osmotic barrier for the egg. This first layer provides the foundation for the successive deposition of two mats of protein fibers. Fibers from both inner and outer layers appear to have similar amino acid compositions and are notably disulfide-rich (comprising about 10% Cys). ESM has been utilized in a wide variety of applications, including nutraceutical supplements, tissue engineering, and nanofabrication, and yet fundamental questions concerning protein composition, fiber structure, and membrane assembly remain to be resolved. We previously identified an abundant disulfide-rich structural protein in chicken ESM fibers (cysteine-rich eggshell membrane protein; CREMP) that contains multiple tandemly repeated modules. In this work, we determine a structural model for four consecutive 2-disulfide containing CREMP modules using a variety of two- and three-dimensional solution NMR experiments. CREMP modules feature an N-terminal loop region positioned above a small beta hairpin that is stabilized by a conserved pattern of disulfide bridges between Cys1-3 and Cys2-4. While the individual CREMP modules are highly ordered, the lack of long-range inter-module restraints suggests an extended structure connected by flexible linkers. Finally, the structural information obtained in this work is considered in the context of full-length CREMP proteins and compared to two other structural proteins that contain multiple tandem repeats of 2-disulfide modules.
- Department of Chemistry and Biochemistry, University of Delaware, Newark, Delaware, USA.
Organizational Affiliation: