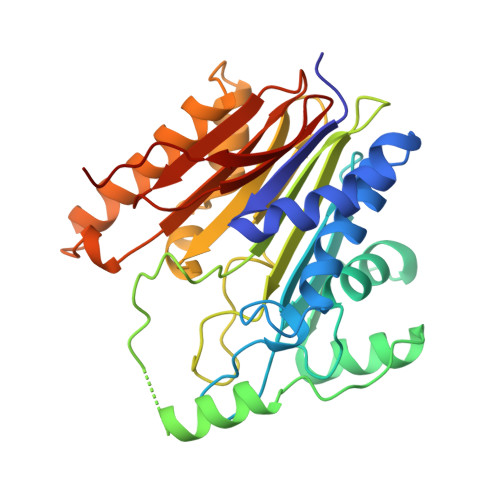
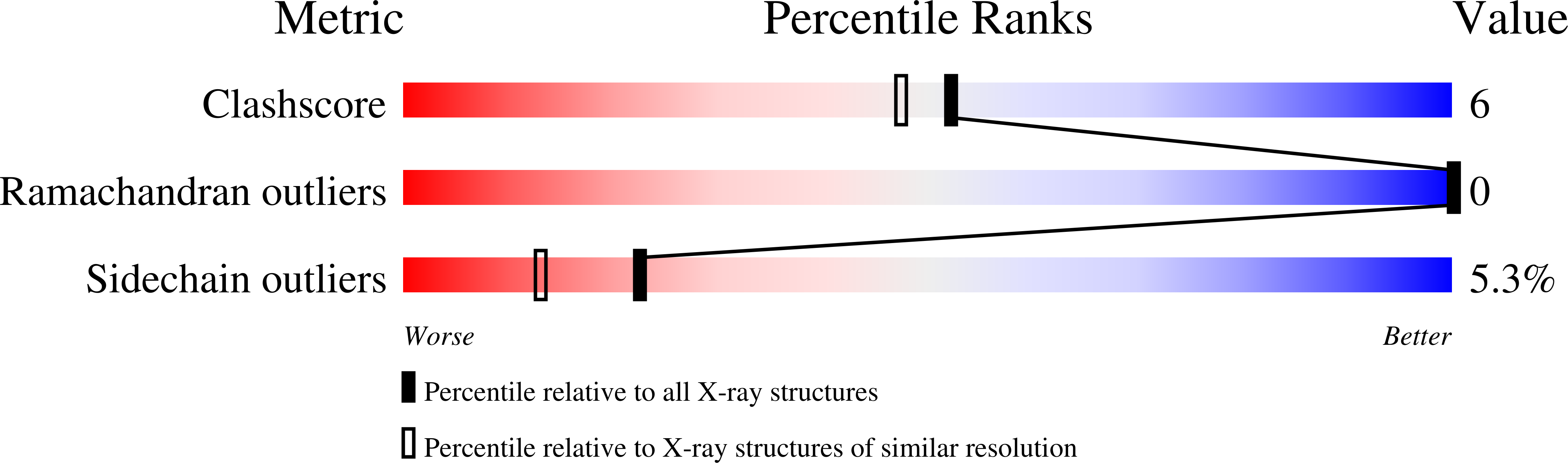Structural insights into the mechanism of intramolecular proteolysis.
Xu, Q., Buckley, D., Guan, C., Guo, H.C.(1999) Cell 98: 651-661
- PubMed: 10490104
- DOI: https://doi.org/10.1016/s0092-8674(00)80052-5
- Primary Citation of Related Structures:
9GAA, 9GAC, 9GAF - PubMed Abstract:
A variety of proteins, including glycosylasparaginase, have recently been found to activate functions by self-catalyzed peptide bond rearrangements from single-chain precursors. Here we present the 1.9 A crystal structures of glycosylasparaginase precursors that are able to autoproteolyze via an N --> O acyl shift. Several conserved residues are aligned around the scissile peptide bond that is in a highly strained trans peptide bond configuration. The structure illustrates how a nucleophilic side chain may attack the scissile peptide bond at the immediate upstream backbone carbonyl and provides an understanding of the structural basis for peptide bond cleavage via an N --> O or N --> S acyl shift that is used by various groups of intramolecular autoprocessing proteins.
Organizational Affiliation:
Department of Biophysics, Boston University School of Medicine, Massachusetts 02118-2526, USA.