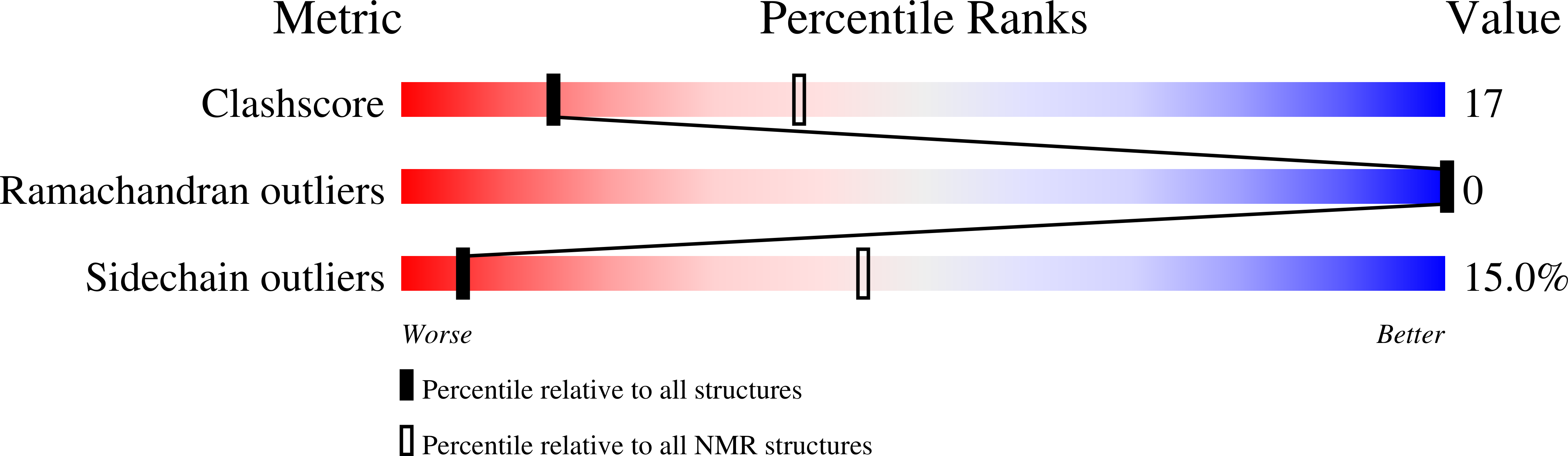Schistocins: Novel antimicrobial peptides encrypted in the Schistosoma mansoni Kunitz Inhibitor SmKI-1.
Santos, B.P.O., Alves, E.S.F., Ferreira, C.S., Ferreira-Silva, A., Goes-Neto, A., Verly, R.M., Liao, L.M., Oliveira, S.C., de Magalhaes, M.T.Q.(2021) Biochim Biophys Acta Gen Subj 1865: 129989-129989
- PubMed: 34389467
- DOI: https://doi.org/10.1016/j.bbagen.2021.129989
- Primary Citation of Related Structures:
7M67, 7M73, 7M77, 7M79 - PubMed Abstract:
Here we describe a new class of cryptides (peptides encrypted within a larger protein) with antimicrobial properties, named schistocins, derived from SmKI-1, a key protein in Shistosoma mansoni survival. This is a multi-functional protein with biotechnological potential usage as a therapeutic molecule in inflammatory diseases and to control schistosomiasis.
Organizational Affiliation:
Laboratório de Biofísica de Macromoléculas, Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais 31270-901, Brazil; Programa Interunidades de Pós-graduação em Bioinformática, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais 31270-901, Brazil.