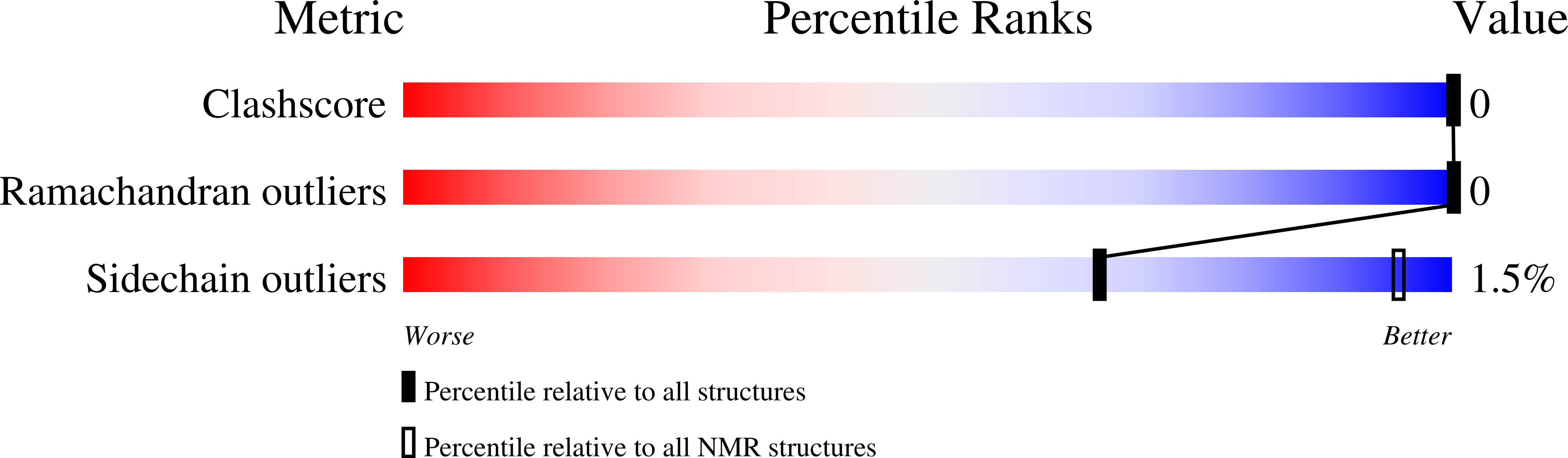Structural Basis of Ca 2+ -Dependent Self-Processing Activity of Repeat-in-Toxin Proteins.
Kuban, V., Macek, P., Hritz, J., Nechvatalova, K., Nedbalcova, K., Faldyna, M., Sebo, P., Zidek, L., Bumba, L.(2020) mBio 11
- PubMed: 32184239
- DOI: https://doi.org/10.1128/mBio.00226-20
- Primary Citation of Related Structures:
6SJW, 6SJX - PubMed Abstract:
The posttranslational Ca 2+ -dependent "clip-and-link" activity of large r epeat-in- t o x in (RTX) proteins starts by Ca 2+ -dependent structural rearrangement of a highly conserved self-processing module (SPM). Subsequently, an internal aspartate-proline (Asp-Pro) peptide bond at the N-terminal end of SPM breaks, and the liberated C-terminal aspartyl residue can react with a free ε-amino group of an adjacent lysine residue to form a new isopeptide bond. Here, we report a solution structure of the calcium-loaded SPM (Ca-SPM) derived from the FrpC protein of Neisseria meningitidis The Ca-SPM structure defines a unique protein architecture and provides structural insight into the autocatalytic cleavage of the Asp-Pro peptide bond through a "twisted-amide" activation. Furthermore, in-frame deletion of the SPM domain from the ApxIVA protein of Actinobacillus pleuropneumoniae attenuated the virulence of this porcine pathogen in a pig respiratory challenge model. We hypothesize that the Ca 2+ -dependent clip-and-link activity represents an unconventional strategy for Gram-negative pathogens to adhere to the host target cell surface. IMPORTANCE The Ca 2+ -dependent clip-and-link activity of large repeat-in-toxin (RTX) proteins is an exceptional posttranslational process in which an internal domain called a self-processing module (SPM) mediates Ca 2+ -dependent processing of a highly specific aspartate-proline (Asp-Pro) peptide bond and covalent linkage of the released aspartyl to an adjacent lysine residue through an isopeptide bond. Here, we report the solution structures of the Ca 2+ -loaded SPM (Ca-SPM) defining the mechanism of the autocatalytic cleavage of the Asp414-Pro415 peptide bond of the Neisseria meningitidis FrpC exoprotein. Moreover, deletion of the SPM domain in the ApxIVA protein, the FrpC homolog of Actinobacillus pleuropneumoniae , resulted in attenuation of virulence of the bacterium in a pig infection model, indicating that the Ca 2+ -dependent clip-and-link activity plays a role in the virulence of Gram-negative pathogens.
Organizational Affiliation:
Central European Institute of Technology, Masaryk University, Brno, Czech Republic.