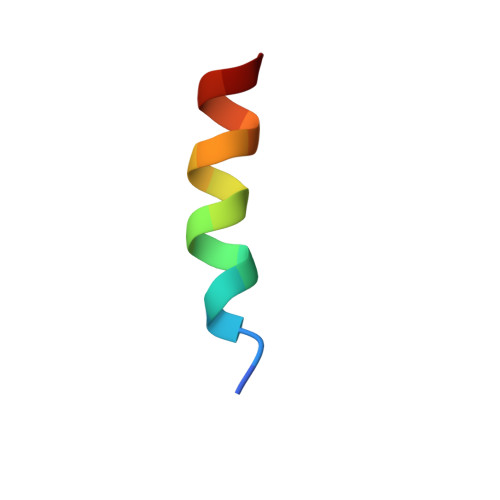
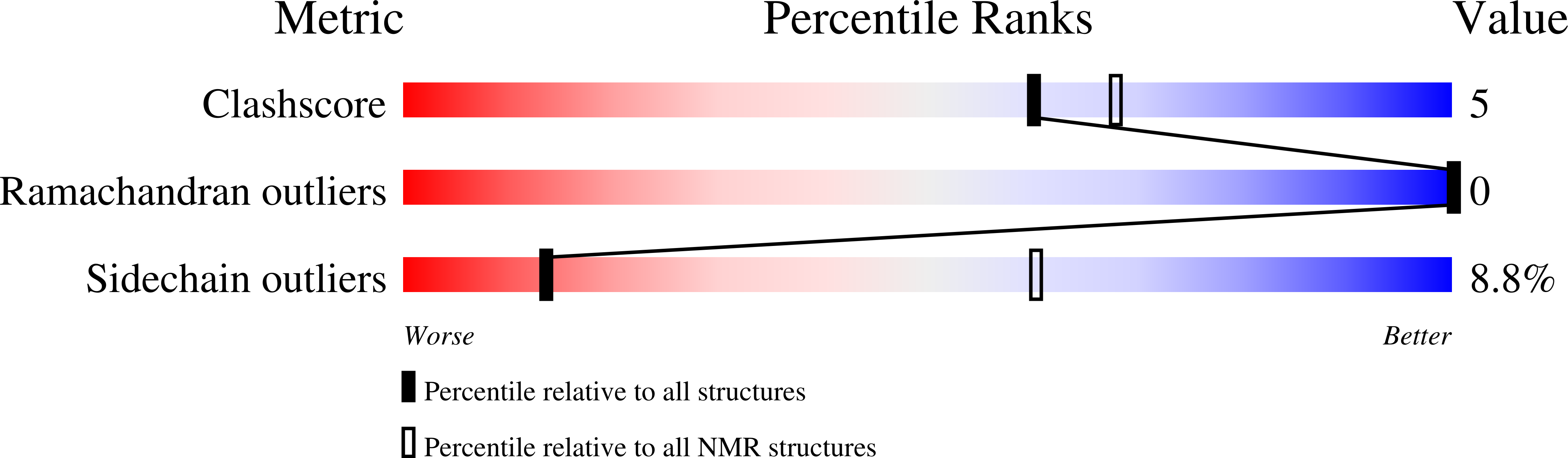Intragenic antimicrobial peptides (IAPs) from human proteins with potent antimicrobial and anti-inflammatory activity.
Brand, G.D., Ramada, M.H.S., Manickchand, J.R., Correa, R., Ribeiro, D.J.S., Santos, M.A., Vasconcelos, A.G., Abrao, F.Y., Prates, M.V., Murad, A.M., Cardozo Fh, J.L., Leite, J.R.S.A., Magalhaes, K.G., Oliveira, A.L., Bloch Jr., C.(2019) PLoS One 14: e0220656-e0220656
- PubMed: 31386688
- DOI: https://doi.org/10.1371/journal.pone.0220656
- Primary Citation of Related Structures:
6MBM - PubMed Abstract:
Following the treads of our previous works on the unveiling of bioactive peptides encrypted in plant proteins from diverse species, the present manuscript reports the occurrence of four proof-of-concept intragenic antimicrobial peptides in human proteins, named Hs IAPs. These IAPs were prospected using the software Kamal, synthesized by solid phase chemistry, and had their interactions with model phospholipid vesicles investigated by differential scanning calorimetry and circular dichroism. Their antimicrobial activity against bacteria, yeasts and filamentous fungi was determined, along with their cytotoxicity towards erythrocytes. Our data demonstrates that Hs IAPs are capable to bind model membranes while attaining α-helical structure, and to inhibit the growth of microorganisms at concentrations as low as 1μM. Hs02, a novel sixteen residue long internal peptide (KWAVRIIRKFIKGFIS-NH2) derived from the unconventional myosin 1h protein, was further investigated in its capacity to inhibit lipopolysaccharide-induced release of TNF-α in murine macrophages. Hs02 presented potent anti-inflammatory activity, inhibiting the release of TNF-α in LPS-primed cells at the lowest assayed concentration, 0.1 μM. A three-dimensional solution structure of Hs02 bound to DPC micelles was determined by Nuclear Magnetic Resonance. Our work exemplifies how the human genome can be mined for molecules with biotechnological potential in human health and demonstrates that IAPs are actual alternatives to antimicrobial peptides as pharmaceutical agents or in their many other putative applications.
Organizational Affiliation:
Laboratório de Síntese e Análise de Biomoléculas, LSAB, Instituto de Química, Universidade de Brasília, Brasília, DF, Brasil.