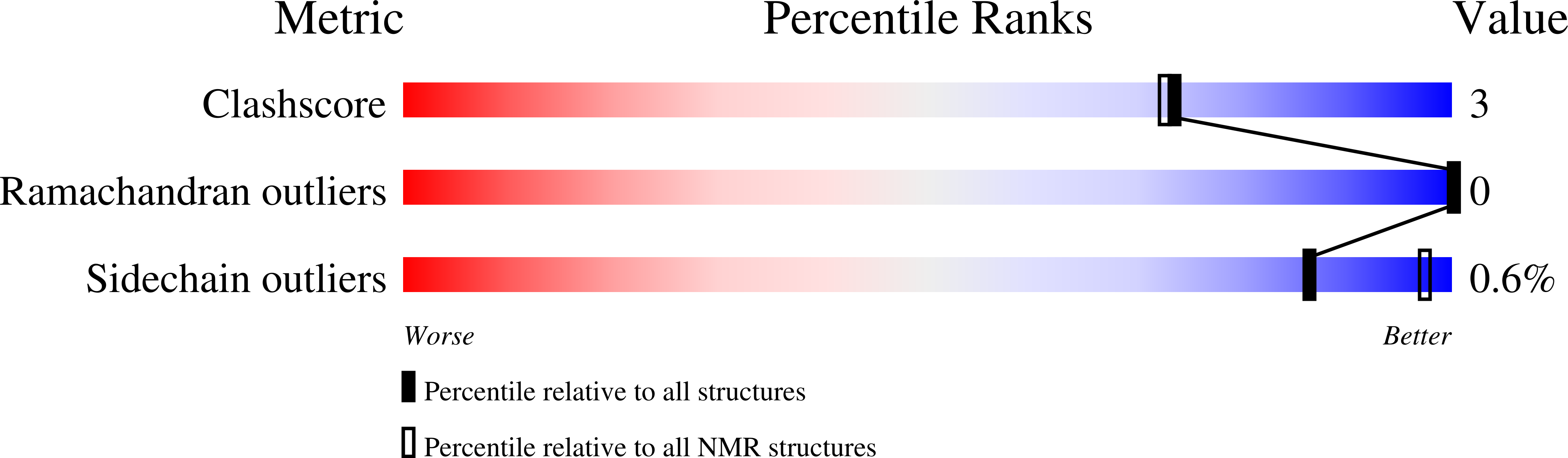Targeted Delivery of Cyclotides via Conjugation to a Nanobody.
Kwon, S., Duarte, J.N., Li, Z., Ling, J.J., Cheneval, O., Durek, T., Schroeder, C.I., Craik, D.J., Ploegh, H.L.(2018) ACS Chem Biol 13: 2973-2980
- PubMed: 30248263
- DOI: https://doi.org/10.1021/acschembio.8b00653
- Primary Citation of Related Structures:
5WOV, 5WOW - PubMed Abstract:
Many naturally occurring peptides have poor proteolytic stability, which limits their therapeutic applications. Cyclotides are plant-derived cyclic peptides that resist proteolysis due to their highly constrained structure, comprising a head-to-tail cyclic backbone and three disulfide bonds that form a cystine-knotted core. This structure makes them useful as scaffolds onto which peptide sequences (epitopes) can be grafted. In this study, VHH7, an alpaca-derived nanobody that targets murine class II MHC molecules, was used for the targeted delivery of cyclotides to antigen-presenting cells (APCs). The cyclotides MCoTI-I, and MCoTI-I with a HA-tag (YPYDVPDYA) grafted into loop 6 (MCoTI-HA), were tested for immunogenic properties. To produce the requisite VHH7-peptide conjugates, a site-specific sortase A-catalyzed reaction in combination with a copper-free strain-promoted cycloaddition reaction was used. MCoTI-I alone did not display any obvious antibody response, thus showing the capacity of cyclotides as immunologically silent scaffolds. By contrast, MCoTI-I conjugated to VHH7 elicited antibodies against cyclic or linear MCoTI-I, thus suggesting a simple and robust approach for targeting cyclotides to APCs, and potentially to other cell types. A similar antibody response was observed when MCoTI-HA was conjugated to VHH7, but there was no reactivity toward a linear HA-tag itself, suggesting differences in conformational constraint between cyclotide-presented and linear epitopes. Studies of commercially available HA antibodies applied to MCoTI-HA confirmed that the conformation of peptide immunogens affects their reactivity. Thus, the production of antibodies that recognize constrained epitopes may benefit from engraftment onto scaffolds such as cyclotides. More broadly, this study validates that a prototypic cyclotide, a member of a peptide family that has proven to be useful as drug design scaffolds in many other studies, can efficiently reach a specific target in vivo.
Organizational Affiliation:
Institute for Molecular Bioscience , The University of Queensland , Brisbane 4072 , Queensland , Australia.