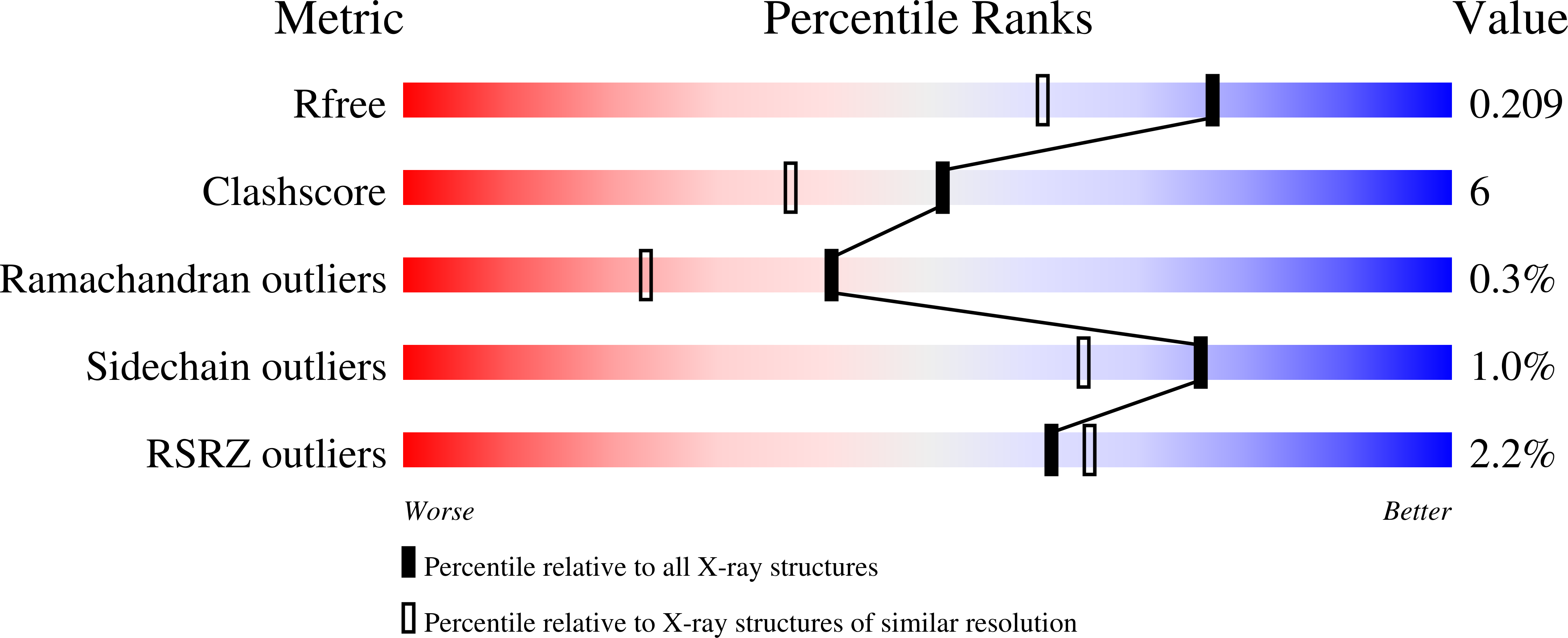
An iterative computational design approach to increase the thermal endurance of a mesophilic enzyme.
Sammond, D.W., Kastelowitz, N., Donohoe, B.S., Alahuhta, M., Lunin, V.V., Chung, D., Sarai, N.S., Yin, H., Mittal, A., Himmel, M.E., Guss, A.M., Bomble, Y.J.(2018) Biotechnol Biofuels 11: 189-189
- PubMed: 30002729
- DOI: https://doi.org/10.1186/s13068-018-1178-9
- Primary Citation of Related Structures:
5TMA - PubMed Abstract:
Strategies for maximizing the microbial production of bio-based chemicals and fuels include eliminating branched points to streamline metabolic pathways. While this is often achieved by removing key enzymes, the introduction of nonnative enzymes can provide metabolic shortcuts, bypassing branched points to decrease the production of undesired side-products. Pyruvate decarboxylase (PDC) can provide such a shortcut in industrially promising thermophilic organisms; yet to date, this enzyme has not been found in any thermophilic organism. Incorporating nonnative enzymes into host organisms can be challenging in cases such as this, where the enzyme has evolved in a very different environment from that of the host.
Organizational Affiliation:
1Biosciences Center, National Renewable Energy Laboratory, 15013 Denver West Parkway, Golden, CO 80401 USA.