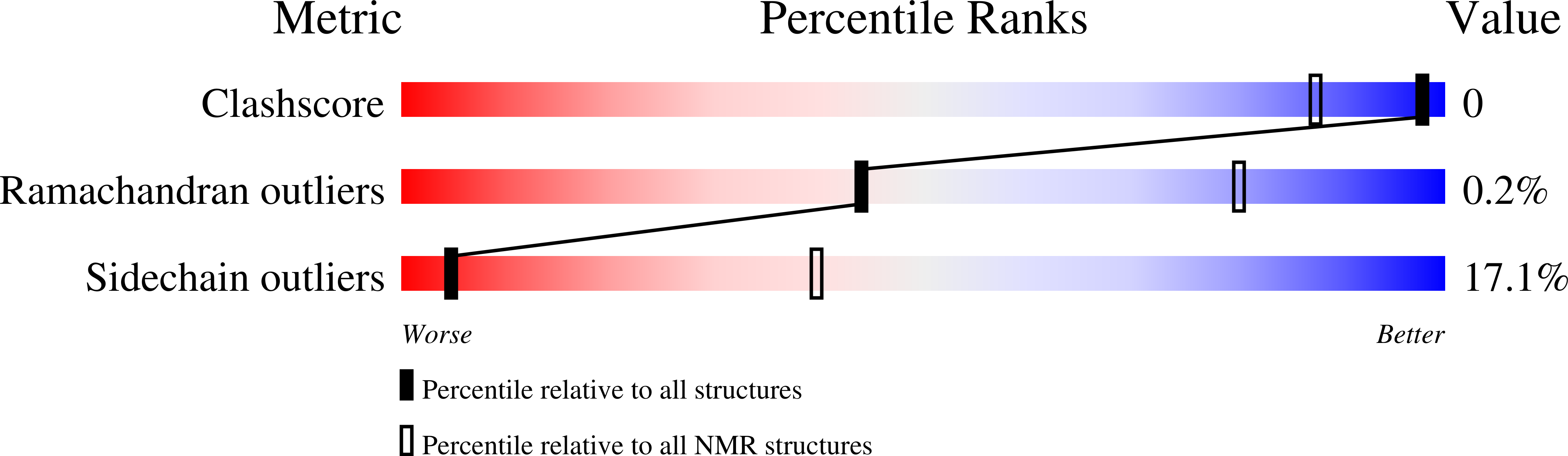Protein aggregation of the p63 transcription factor underlies severe skin fragility in AEC syndrome.
Russo, C., Osterburg, C., Sirico, A., Antonini, D., Ambrosio, R., Wurz, J.M., Rinnenthal, J., Ferniani, M., Kehrloesser, S., Schafer, B., Guntert, P., Sinha, S., Dotsch, V., Missero, C.(2018) Proc Natl Acad Sci U S A 115: E906-E915
- PubMed: 29339502
- DOI: https://doi.org/10.1073/pnas.1713773115
- Primary Citation of Related Structures:
5N2O - PubMed Abstract:
The p63 gene encodes a master regulator of epidermal commitment, development, and differentiation. Heterozygous mutations in the C-terminal domain of the p63 gene can cause ankyloblepharon-ectodermal defects-cleft lip/palate (AEC) syndrome, a life-threatening disorder characterized by skin fragility and severe, long-lasting skin erosions. Despite deep knowledge of p63 functions, little is known about mechanisms underlying disease pathology and possible treatments. Here, we show that multiple AEC-associated p63 mutations, but not those causative of other diseases, lead to thermodynamic protein destabilization, misfolding, and aggregation, similar to the known p53 gain-of-function mutants found in cancer. AEC mutant proteins exhibit impaired DNA binding and transcriptional activity, leading to dominant negative effects due to coaggregation with wild-type p63 and p73. Importantly, p63 aggregation occurs also in a conditional knock-in mouse model for the disorder, in which the misfolded p63 mutant protein leads to severe epidermal defects. Variants of p63 that abolish aggregation of the mutant proteins are able to rescue p63's transcriptional function in reporter assays as well as in a human fibroblast-to-keratinocyte conversion assay. Our studies reveal that AEC syndrome is a protein aggregation disorder and opens avenues for therapeutic intervention.
Organizational Affiliation:
Centro di Ingegneria Gentica e Biotecnologie Avanzate, 80145 Naples, Italy.