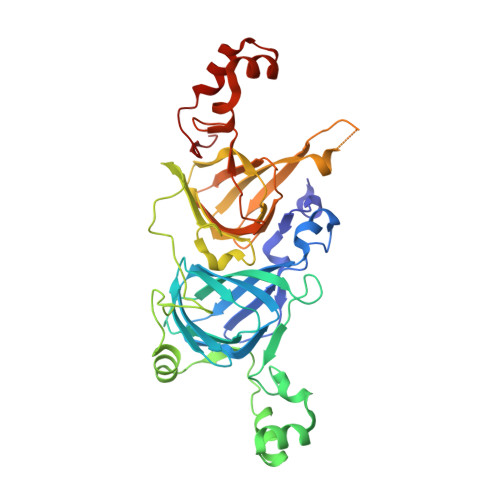
Crystal structure of the vicilin from Solanum melongena reveals existence of different anionic ligands in structurally similar pockets
Jain, A., Kumar, A., Salunke, D.M.(2016) Sci Rep 6: 23600-23600
- PubMed: 27004988
- DOI: https://doi.org/10.1038/srep23600
- Primary Citation of Related Structures:
5CAD - PubMed Abstract:
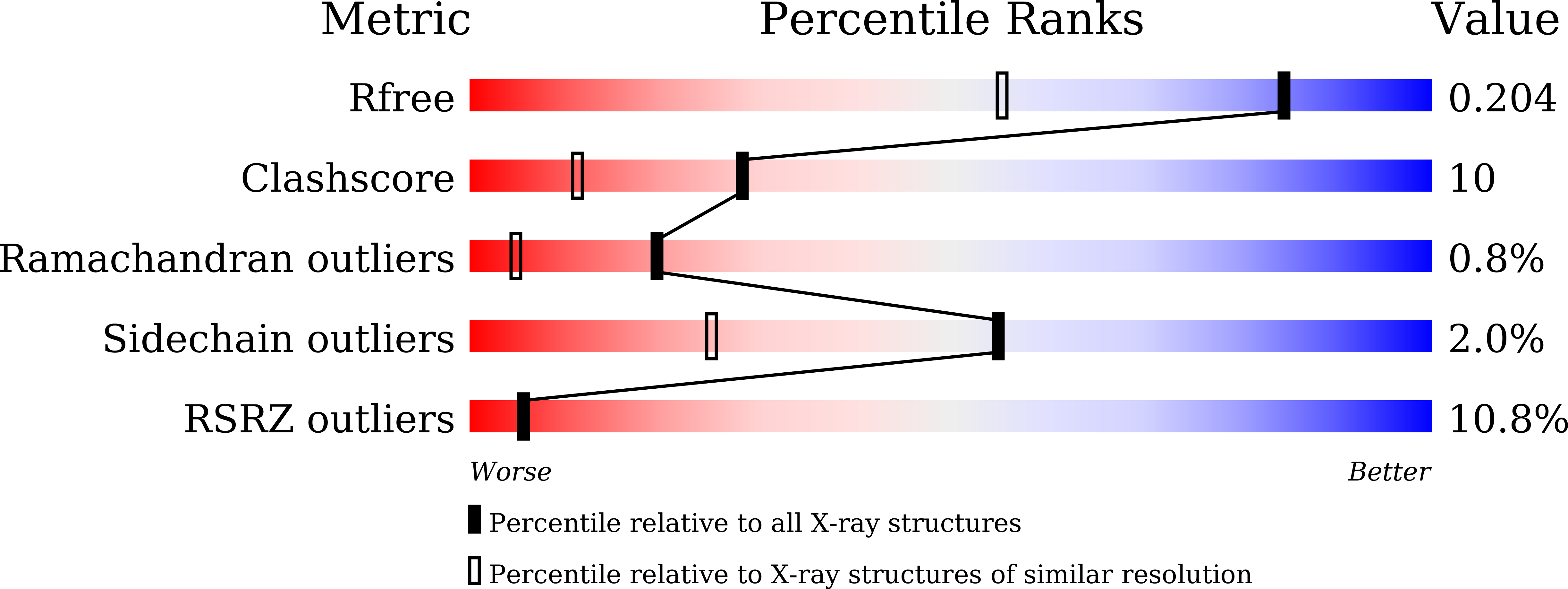
Crystal structure of a vicilin, SM80.1, was determined towards exploring its possible physiological functions. The protein was purified from Solanum melongena by combination of ammonium sulphate fractionation and size exclusion chromatography. Structure was determined ab initio at resolution of 1.5 Å by X-ray crystallography showing the three-dimensional topology of the trimeric protein. Each monomer of SM80.1 consists of two similar domains with hydrophobic binding pocket and each accommodating different ligands, i.e. acetate and pyroglutamate. The relatively high stability of these independent anionic ligands in similar pockets indicated a strict requirement of stabilization by hydrogen bonds with the charged residues, suggesting a degree of plasticity within the binding pocket. Comparison of SM80.1 structure with those of other 7S vicilins indicated conservation of putative binding pocket for anionic ligands. Here we propose the possibility of trapping of these ligands in the protein for their requirement in the metabolic processes.
Organizational Affiliation:
Regional Centre for Biotechnology, Faridabad-121001, India.