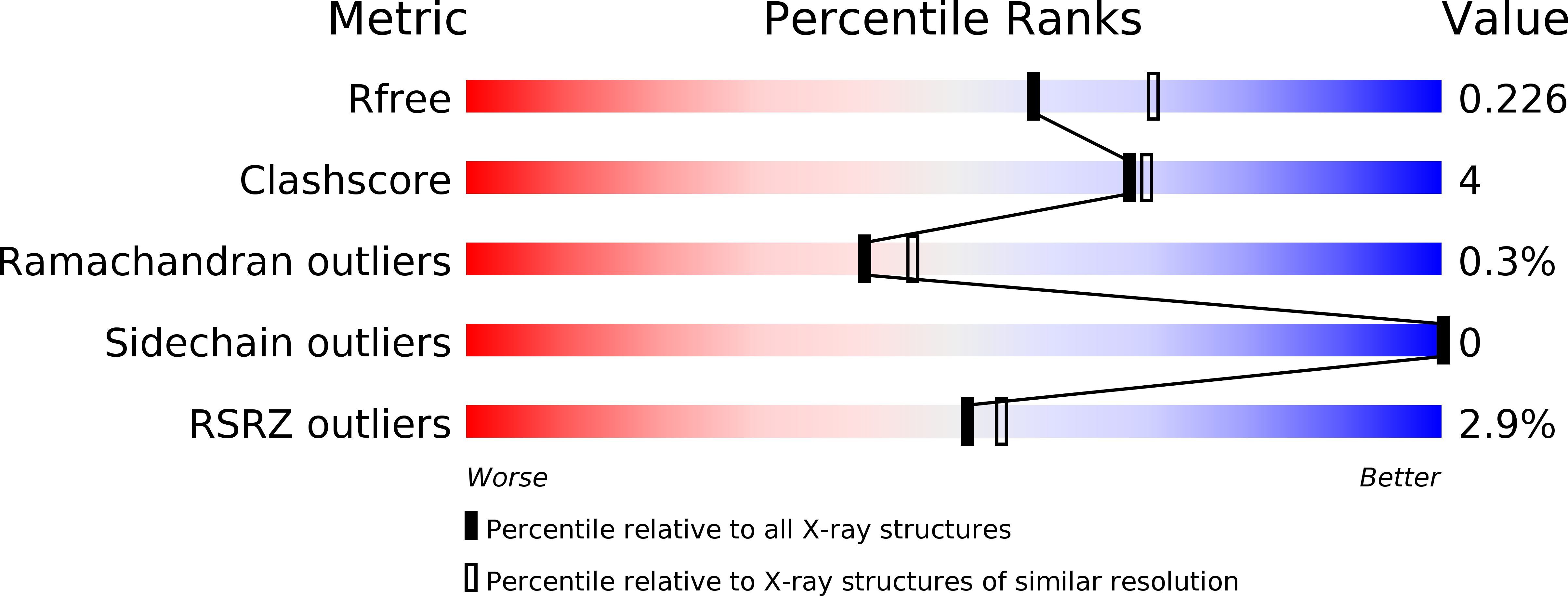
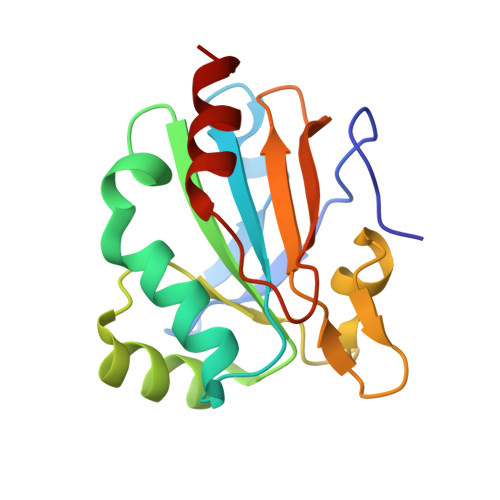
The active site architecture in peroxiredoxins: a case study on Mycobacterium tuberculosis AhpE.
Pedre, B., van Bergen, L.A., Pallo, A., Rosado, L.A., Dufe, V.T., Molle, I.V., Wahni, K., Erdogan, H., Alonso, M., Proft, F.D., Messens, J.(2016) Chem Commun (Camb) 52: 10293-10296
- PubMed: 27471753
- DOI: https://doi.org/10.1039/c6cc02645a
- Primary Citation of Related Structures:
4XIH, 5C04 - PubMed Abstract:
Peroxiredoxins catalyze the reduction of peroxides, a process of vital importance to survive oxidative stress. A nucleophilic cysteine, also known as the peroxidatic cysteine, is responsible for this catalytic process. We used the Mycobacterium tuberculosis alkyl hydroperoxide reductase E (MtAhpE) as a model to investigate the effect of the chemical environment on the specificity of the reaction. Using an integrative structural (R116A - PDB ; F37H - PDB ), kinetic and computational approach, we explain the mutational effects of key residues in its environment. This study shows that the active site residues are specifically oriented to create an environment which selectively favours a reaction with peroxides.
Organizational Affiliation:
Structural Biology Research Center, Oxidative Stress Signaling lab, VIB, Pleinlaan 2, 1050 Brussels, Belgium. joris.messens@vib-vub.be and Brussels Center for Redox Biology, 1050 Brussels, Belgium and Structural Biology Brussels, Vrije Universiteit Brussel, 1050 Brussels, Belgium.