Production in Pichia pastoris, antifungal activity and crystal structure of a class I chitinase from cowpea (Vigna unguiculata): Insights into sugar binding mode and hydrolytic action.
Landim, P.G., Correia, T.O., Silva, F.D., Nepomuceno, D.R., Costa, H.P., Pereira, H.M., Lobo, M.D., Moreno, F.B., Brandao-Neto, J., Medeiros, S.C., Vasconcelos, I.M., Oliveira, J.T., Sousa, B.L., Barroso-Neto, I.L., Freire, V.N., Carvalho, C.P., Monteiro-Moreira, A.C., Grangeiro, T.B.(2017) Biochimie
- PubMed: 28153694
- DOI: https://doi.org/10.1016/j.biochi.2017.01.014
- Primary Citation of Related Structures:
4TX7 - PubMed Abstract:
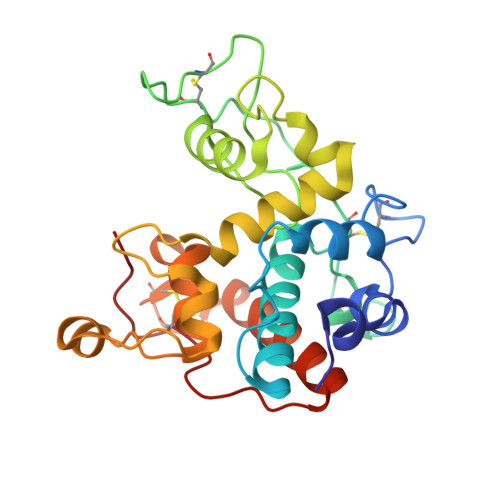
A cowpea class I chitinase (VuChiI) was expressed in the methylotrophic yeast P. pastoris. The recombinant protein was secreted into the culture medium and purified by affinity chromatography on a chitin matrix. The purified chitinase migrated on SDS-polyacrylamide gel electrophoresis as two closely-related bands with apparent molecular masses of 34 and 37 kDa. The identity of these bands as VuChiI was demonstrated by mass spectrometry analysis of tryptic peptides and N-terminal amino acid sequencing. The recombinant chitinase was able to hydrolyze colloidal chitin but did not exhibit enzymatic activity toward synthetic substrates. The highest hydrolytic activity of the cowpea chitinase toward colloidal chitin was observed at pH 5.0. Furthermore, most VuChiI activity (approximately 92%) was retained after heating to 50 °C for 30 min, whereas treatment with 5 mM Cu 2+ caused a reduction of 67% in the enzyme's chitinolytic activity. The recombinant protein had antifungal activity as revealed by its ability to inhibit the spore germination and mycelial growth of Penicillium herquei. The three-dimensional structure of VuChiI was resolved at a resolution of 1.55 Å by molecular replacement. The refined model had 245 amino acid residues and 381 water molecules, and the final R-factor and R free values were 14.78 and 17.22%, respectively. The catalytic domain of VuChiI adopts an α-helix-rich fold, stabilized by 3 disulfide bridges and possessing a wide catalytic cleft. Analysis of the crystallographic model and molecular docking calculations using chito-oligosaccharides provided evidences about the VuChiI residues involved in sugar binding and catalysis, and a possible mechanism of antifungal action is suggested.
Organizational Affiliation:
Laboratório de Genética Molecular, Departamento de Biologia, Centro de Ciências, Universidade Federal do Ceará, Fortaleza, Ceará, Brazil.