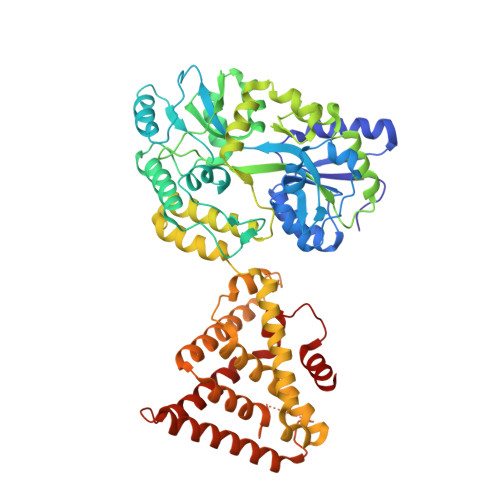
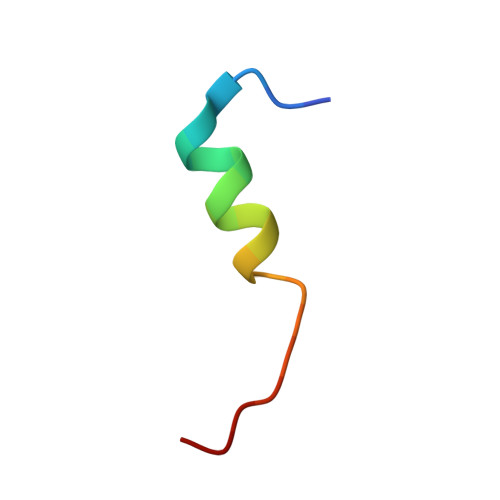
Structural basis for corepressor assembly by the orphan nuclear receptor TLX.
Zhi, X., Zhou, X.E., He, Y., Searose-Xu, K., Zhang, C.L., Tsai, C.C., Melcher, K., Xu, H.E.(2015) Genes Dev 29: 440-450
- PubMed: 25691470
- DOI: https://doi.org/10.1101/gad.254904.114
- Primary Citation of Related Structures:
4XAI, 4XAJ - PubMed Abstract:
The orphan nuclear receptor TLX regulates neural stem cell self-renewal in the adult brain and functions primarily as a transcription repressor through recruitment of Atrophin corepressors, which bind to TLX via a conserved peptide motif termed the Atro box. Here we report crystal structures of the human and insect TLX ligand-binding domain in complex with Atro box peptides. In these structures, TLX adopts an autorepressed conformation in which its helix H12 occupies the coactivator-binding groove. Unexpectedly, H12 in this autorepressed conformation forms a novel binding pocket with residues from helix H3 that accommodates a short helix formed by the conserved ALXXLXXY motif of the Atro box. Mutations that weaken the TLX-Atrophin interaction compromise the repressive activity of TLX, demonstrating that this interaction is required for Atrophin to confer repressor activity to TLX. Moreover, the autorepressed conformation is conserved in the repressor class of orphan nuclear receptors, and mutations of corresponding residues in other members of this class of receptors diminish their repressor activities. Together, our results establish the functional conservation of the autorepressed conformation and define a key sequence motif in the Atro box that is essential for TLX-mediated repression.
Organizational Affiliation:
Laboratory of Structural Sciences, Van Andel Research Institute, Grand Rapids, Michigan 49503, USA; Autophagy Research Center, eric.xu@vai.org xiaoyong.zhi@utsouthwestern.edu.