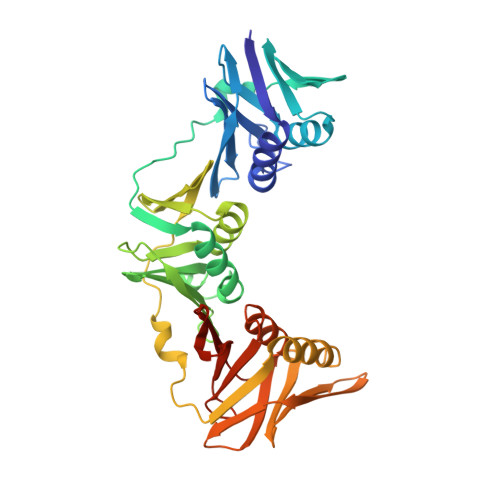
Crystal structure of the DNA polymerase III beta subunit ( beta-clamp) from the extremophile Deinococcus radiodurans.
Niiranen, L., Lian, K., Johnson, K.A., Moe, E.(2015) BMC Struct Biol 15: 5-5
- PubMed: 25886944
- DOI: https://doi.org/10.1186/s12900-015-0032-6
- Primary Citation of Related Structures:
4TRT - PubMed Abstract:
Deinococcus radiodurans is an extremely radiation and desiccation resistant bacterium which can tolerate radiation doses up to 5,000 Grays without losing viability. We are studying the role of DNA repair and replication proteins for this unusual phenotype by a structural biology approach. The DNA polymerase III β subunit (β-clamp) acts as a sliding clamp on DNA, promoting the binding and processivity of many DNA-acting proteins, and here we report the crystal structure of D. radiodurans β-clamp (Drβ-clamp) at 2.0 Å resolution.
Organizational Affiliation:
The Norwegian Structural Biology Center (NorStruct), Department of Chemistry, UIT - the Arctic University of Norway, N-9037, Tromsø, Norway. laila.niiranen@live.fi.