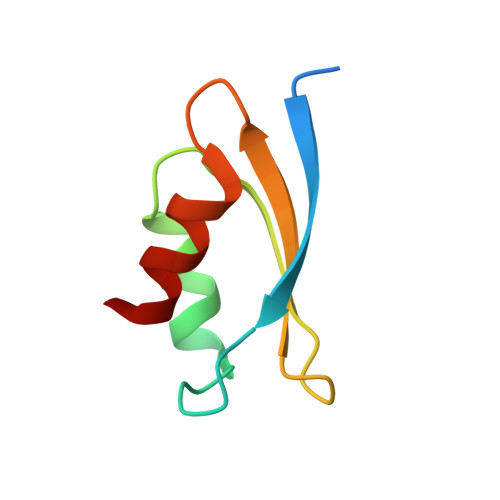
Atomic resolution structure of the E. coli YajR transporter YAM domain.
Jiang, D., Zhao, Y., Fan, J., Liu, X., Wu, Y., Feng, W., Zhang, X.C.(2014) Biochem Biophys Res Commun 450: 929-935
- PubMed: 24952155
- DOI: https://doi.org/10.1016/j.bbrc.2014.06.053
- Primary Citation of Related Structures:
2RU9, 4Q2L, 4Q2M - PubMed Abstract:
YajR is an Escherichia coli transporter that belongs to the major facilitator superfamily. Unlike most MFS transporters, YajR contains a carboxyl terminal, cytosolic domain of 67 amino acid residues termed YAM domain. Although it is speculated that the function of this small soluble domain is to regulate the conformational change of the 12-helix transmembrane domain, its precise regulatory role remains unclear. Here, we report the crystal structure of the YAM domain at 1.07-Å resolution, along with its structure determined using nuclear magnetic resonance. Detailed analysis of the high resolution structure revealed a symmetrical dimer in which a belt of well-ordered poly-pentagonal water molecules is embedded. A mutagenesis experiment and a thermal stability assay were used to analyze the putative role of this dimerization in response to changes in halogen concentration.
Organizational Affiliation:
National Laboratory of Macromolecules, National Center of Protein Science-Beijing, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Beijing 100101, China; School of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, Hubei 430074, China.