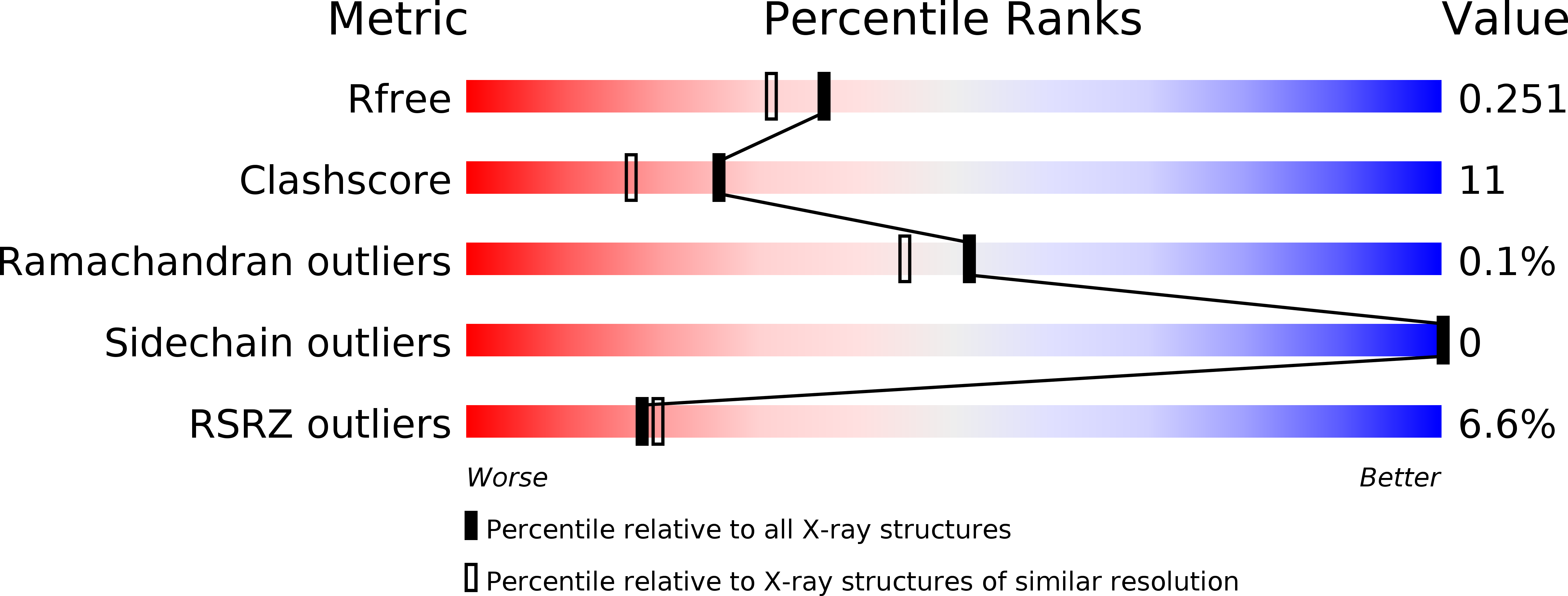
Structure and function of a TetR family transcriptional regulator, SbtR, from thermus thermophilus HB8
Agari, Y., Sakamoto, K., Yutani, K., Kuramitsu, S., Shinkai, A.(2013) Proteins 81: 1166-1178
- PubMed: 23408580
- DOI: https://doi.org/10.1002/prot.24266
- Primary Citation of Related Structures:
3VUQ - PubMed Abstract:
SbtR is one of the four TetR family transcriptional regulators present in the extremely thermophilic bacterium, Thermus thermophilus HB8. We identified 10 genes controlled by four promoters with negative regulation by SbtR in vitro. The SbtR-regulated gene products include probable transporters, probable enzymes for sugar or amino acid metabolism, and nucleic acid-related enzymes. SbtR binds pseudopalindromic sequences, with the consensus sequence of 5'-TGACCCNNKGGTCA-3' surrounding the promoters, and has a proposed 1:1 dimer binding stoichiometry. The X-ray crystal structure analysis revealed that SbtR comprises either nine or 10 α-helices and forms a dimer, as in the typical TetR family proteins. Similar to many characterized TetR family regulators, SbtR has a predicted ligand-binding pocket at the center of each monomer. Interestingly, the SbtR dimer contains an intermolecular disulfide bridge, formed between the Cys164 residues at the entrance of the pocket. The Cys164Ser and Cys164Ala mutant SbtR proteins formed homodimers similar to that of the wild type, but their thermal stabilities were lower by about 8°C, indicating that the disulfide bridge contributes to the thermal stability of the protein. However, altered repression activity of the mutants was not observed in vitro. From these results, we propose that ligand-binding is essential for SbtR to disengage from DNA, in a similar manner to the other characterized TetR family regulators. The formation and reduction of the disulfide bond might function in controlling the ligand-binding affinity of this transcriptional regulator.
Organizational Affiliation:
RIKEN SPring-8 Center, RIKEN Harima Institute, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan.