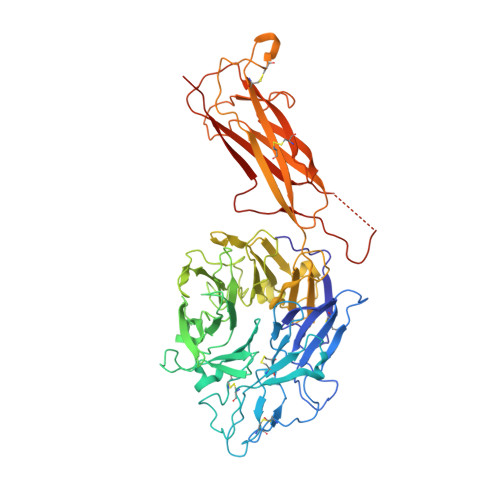
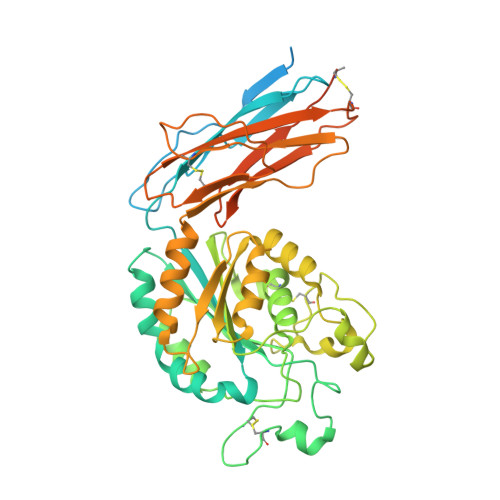
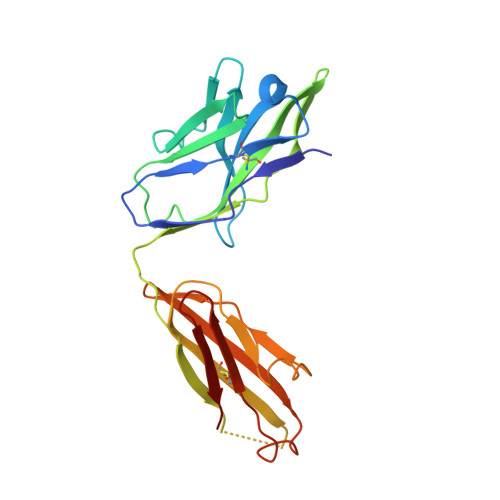
Structural specializations of a4b7, an Integrin that Mediates Rolling Adhesion
Yu, Y., Zhu, J., Mi, L.Z., Walz, T., Sun, H., Chen, J., Springer, T.A.(2012) J Cell Biol 196: 131-146
- PubMed: 22232704
- DOI: https://doi.org/10.1083/jcb.201110023
- Primary Citation of Related Structures:
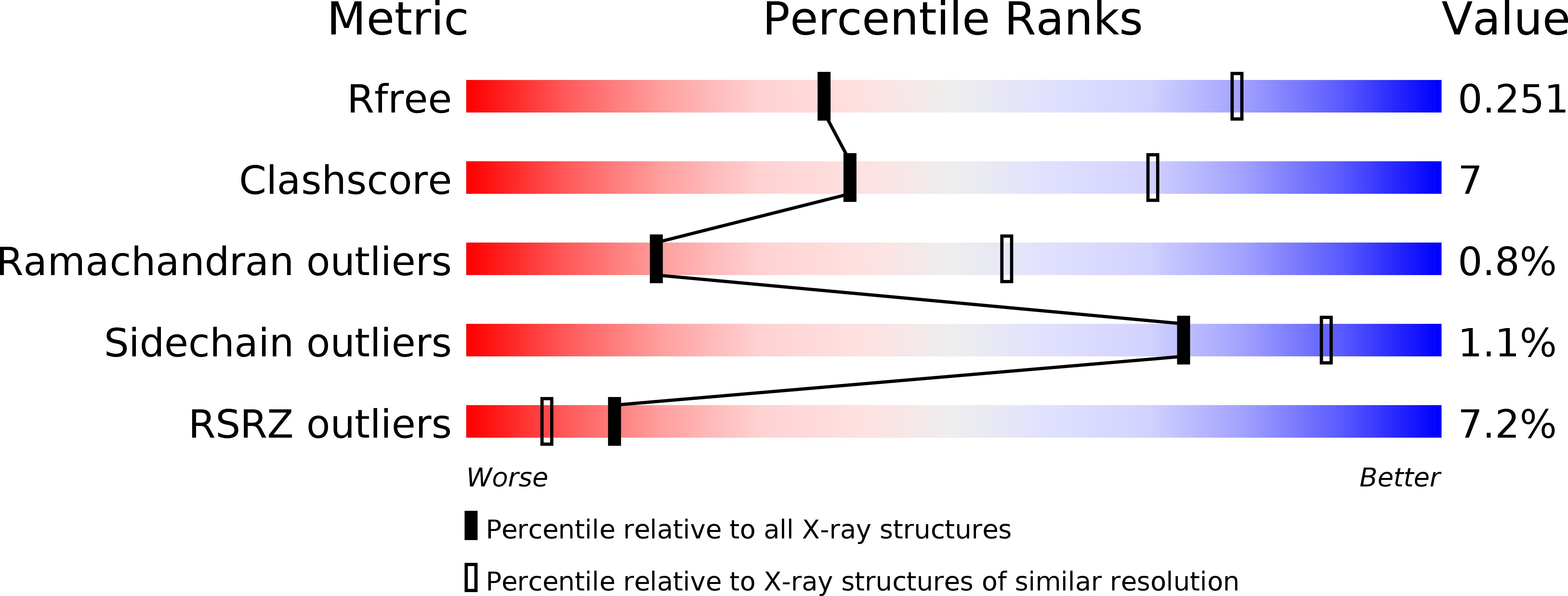
3V4P, 3V4V - PubMed Abstract:
The lymphocyte homing receptor integrin α(4)β(7) is unusual for its ability to mediate both rolling and firm adhesion. α(4)β(1) and α(4)β(7) are targeted by therapeutics approved for multiple sclerosis and Crohn's disease. Here, we show by electron microscopy and crystallography how two therapeutic Fabs, a small molecule (RO0505376), and mucosal adhesion molecule-1 (MAdCAM-1) bind α(4)β(7). A long binding groove at the α(4)-β(7) interface for immunoglobulin superfamily domains differs in shape from integrin pockets that bind Arg-Gly-Asp motifs. RO0505376 mimics an Ile/Leu-Asp motif in α(4) ligands, and orients differently from Arg-Gly-Asp mimics. A novel auxiliary residue at the metal ion-dependent adhesion site in α(4)β(7) is essential for binding to MAdCAM-1 in Mg(2+) yet swings away when RO0505376 binds. A novel intermediate conformation of the α(4)β(7) headpiece binds MAdCAM-1 and supports rolling adhesion. Lack of induction of the open headpiece conformation by ligand binding enables rolling adhesion to persist until integrin activation is signaled.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Immune Disease Institute and Children's Hospital, Boston, MA 02115, USA.