The structure of native bacterial chemoreceptor arrays
Breigel, A., Li, X., Bilwes, A.M., Hugues, K.T., Jensen, G.J., Crane, B.R.(2012) Proc Natl Acad Sci U S A
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2012) Proc Natl Acad Sci U S A
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Chemotaxis protein CheA | 320 | Thermotoga maritima | Mutation(s): 0 Gene Names: cheA, TM_0702 EC: 2.7.13.3 | 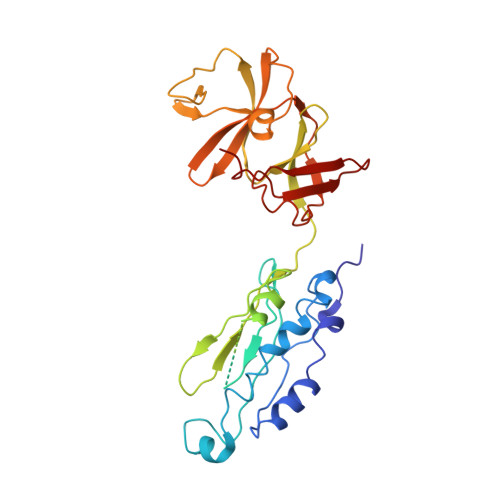 | |
UniProt | |||||
Find proteins for Q56310 (Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8)) Explore Q56310 Go to UniProtKB: Q56310 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q56310 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Chemotaxis protein CheW | 139 | Thermotoga maritima | Mutation(s): 0 Gene Names: cheW, TM_0701 | 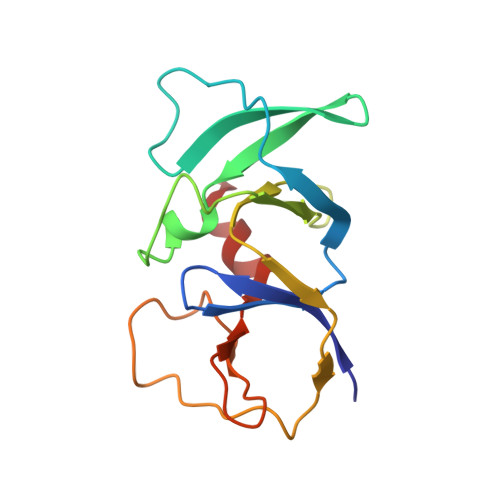 | |
UniProt | |||||
Find proteins for Q56311 (Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8)) Explore Q56311 Go to UniProtKB: Q56311 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q56311 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Methyl-accepting chemotaxis protein | 85 | Thermotoga maritima | Mutation(s): 0 Gene Names: TM_0014 | 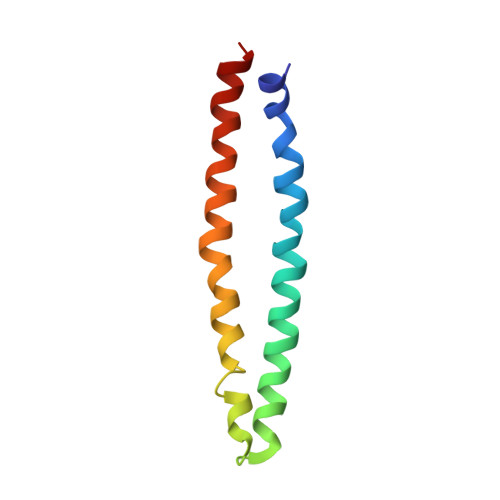 | |
UniProt | |||||
Find proteins for Q7DFA3 (Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8)) Explore Q7DFA3 Go to UniProtKB: Q7DFA3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7DFA3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 213.991 | α = 90 |
| b = 213.991 | β = 90 |
| c = 208.192 | γ = 120 |
| Software Name | Purpose |
|---|---|
| ADSC | data collection |
| PHASER | phasing |
| CNS | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |