In vitro and in vivo studies of the trypanocidal properties of WRR-483 against Trypanosoma cruzi.
Chen, Y.T., Brinen, L.S., Kerr, I.D., Hansell, E., Doyle, P.S., McKerrow, J.H., Roush, W.R.(2010) PLoS Negl Trop Dis 4
- PubMed: 20856868
- DOI: https://doi.org/10.1371/journal.pntd.0000825
- Primary Citation of Related Structures:
3LXS - PubMed Abstract:
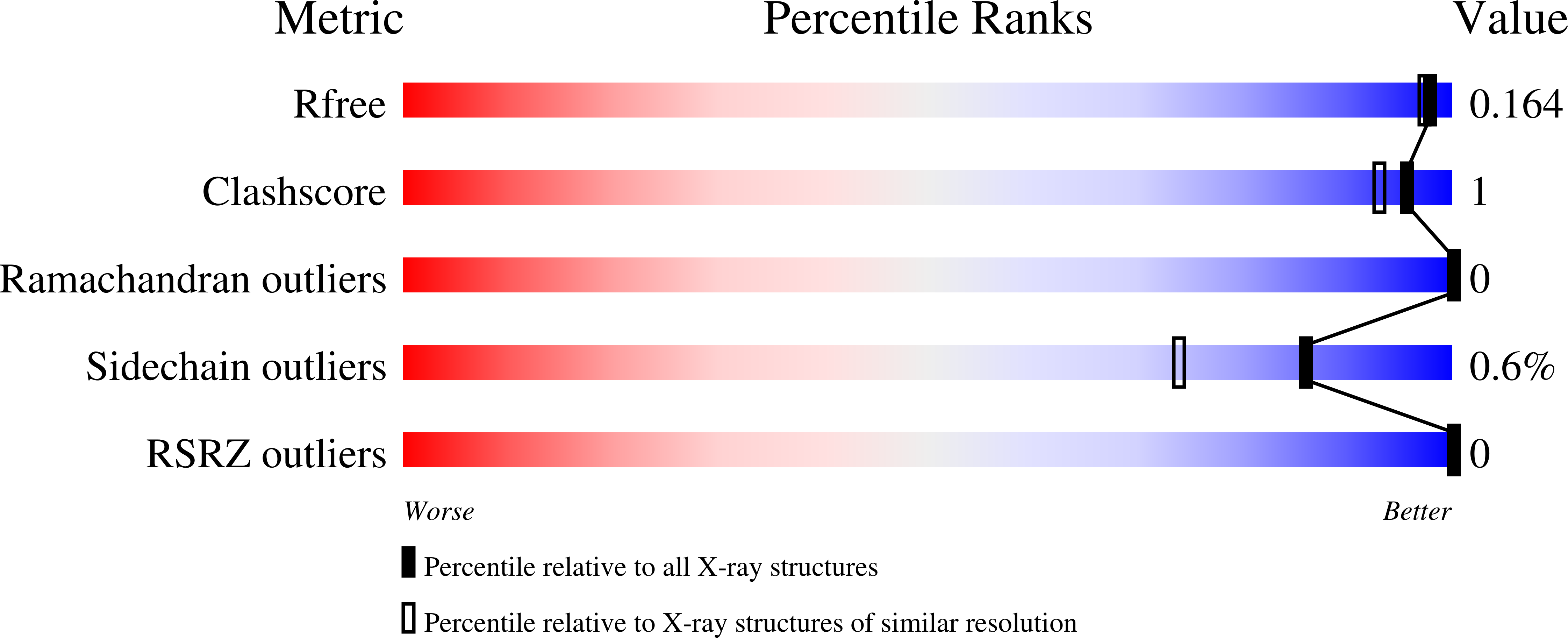
Cruzain, the major cysteine protease of Trypanosoma cruzi, is an essential enzyme for the parasite life cycle and has been validated as a viable target to treat Chagas' disease. As a proof-of-concept, K11777, a potent inhibitor of cruzain, was found to effectively eliminate T. cruzi infection and is currently a clinical candidate for treatment of Chagas' disease. WRR-483, an analog of K11777, was synthesized and evaluated as an inhibitor of cruzain and against T. cruzi proliferation in cell culture. This compound demonstrates good potency against cruzain with sensitivity to pH conditions and high efficacy in the cell culture assay. Furthermore, WRR-483 also eradicates parasite infection in a mouse model of acute Chagas' disease. To determine the atomic-level details of the inhibitor interacting with cruzain, a 1.5 A crystal structure of the protease in complex with WRR-483 was solved. The structure illustrates that WRR-483 binds covalently to the active site cysteine of the protease in a similar manner as other vinyl sulfone-based inhibitors. Details of the critical interactions within the specificity binding pocket are also reported. We demonstrate that WRR-483 is an effective cysteine protease inhibitor with trypanocidal activity in cell culture and animal model with comparable efficacy to K11777. Crystallographic evidence confirms that the mode of action is by targeting the active site of cruzain. Taken together, these results suggest that WRR-483 has potential to be developed as a treatment for Chagas' disease.
Organizational Affiliation:
Department of Chemistry, The Scripps Research Institute, Scripps Florida, Jupiter, Florida, United States of America.