Dcn1 Functions as a Scaffold-Type E3 Ligase for Cullin Neddylation.
Kurz, T., Chou, Y.C., Willems, A.R., Meyer-Schaller, N., Hecht, M.L., Tyers, M., Peter, M., Sicheri, F.(2008) Mol Cell 29: 23-35
- PubMed: 18206966
- DOI: https://doi.org/10.1016/j.molcel.2007.12.012
- Primary Citation of Related Structures:
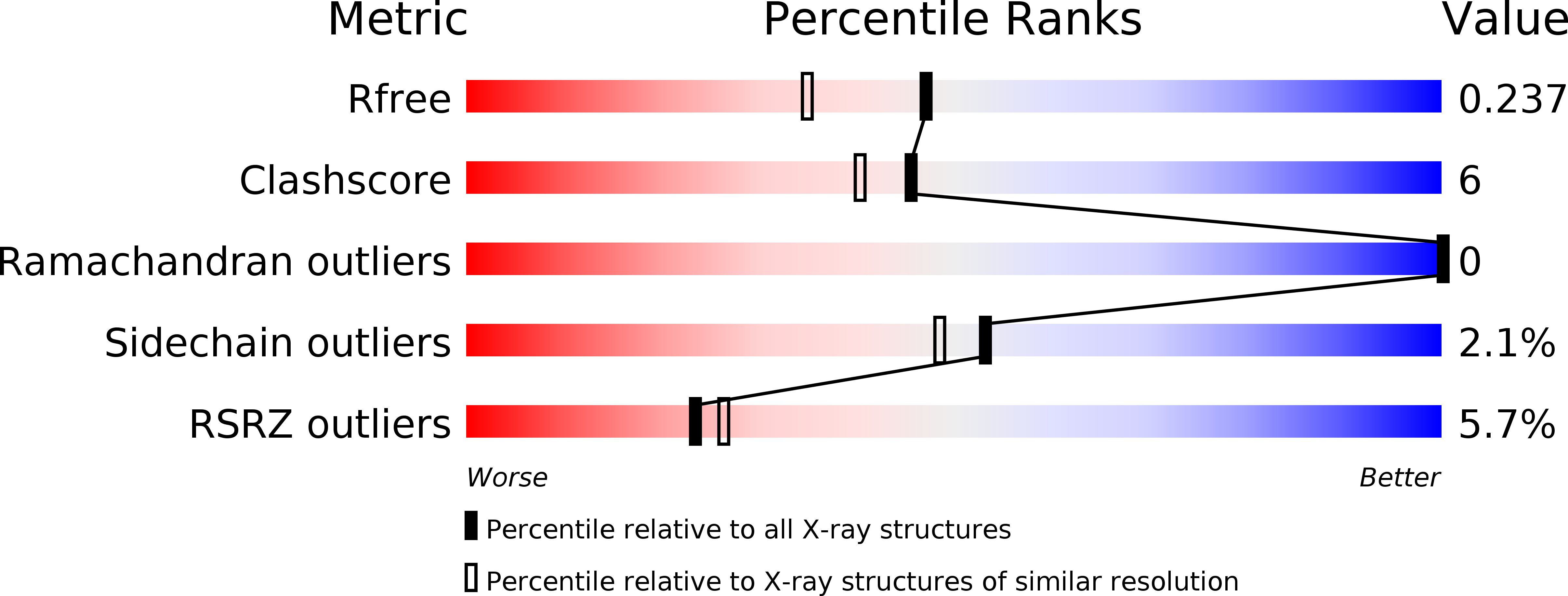
3BQ3 - PubMed Abstract:
Cullin-based E3 ubiquitin ligases are activated through modification of the cullin subunit with the ubiquitin-like protein Nedd8. Dcn1 regulates cullin neddylation and thus ubiquitin ligase activity. Here we describe the 1.9 A X-ray crystal structure of yeast Dcn1 encompassing an N-terminal ubiquitin-binding (UBA) domain and a C-terminal domain of unique architecture, which we termed PONY domain. A conserved surface on Dcn1 is required for direct binding to cullins and for neddylation. The reciprocal binding site for Dcn1 on Cdc53 is located approximately 18 A from the site of neddylation. Dcn1 does not require cysteine residues for catalytic function, and directly interacts with the Nedd8 E2 Ubc12 on a surface that overlaps with the E1-binding site. We show that Dcn1 is necessary and sufficient for cullin neddylation in a purified recombinant system. Taken together, these data demonstrate that Dcn1 is a scaffold-like E3 ligase for cullin neddylation.
Organizational Affiliation:
Institute of Biochemistry, ETH Zürich, Schafmattstrasse 18, CH-8093 Zürich, Switzerland.