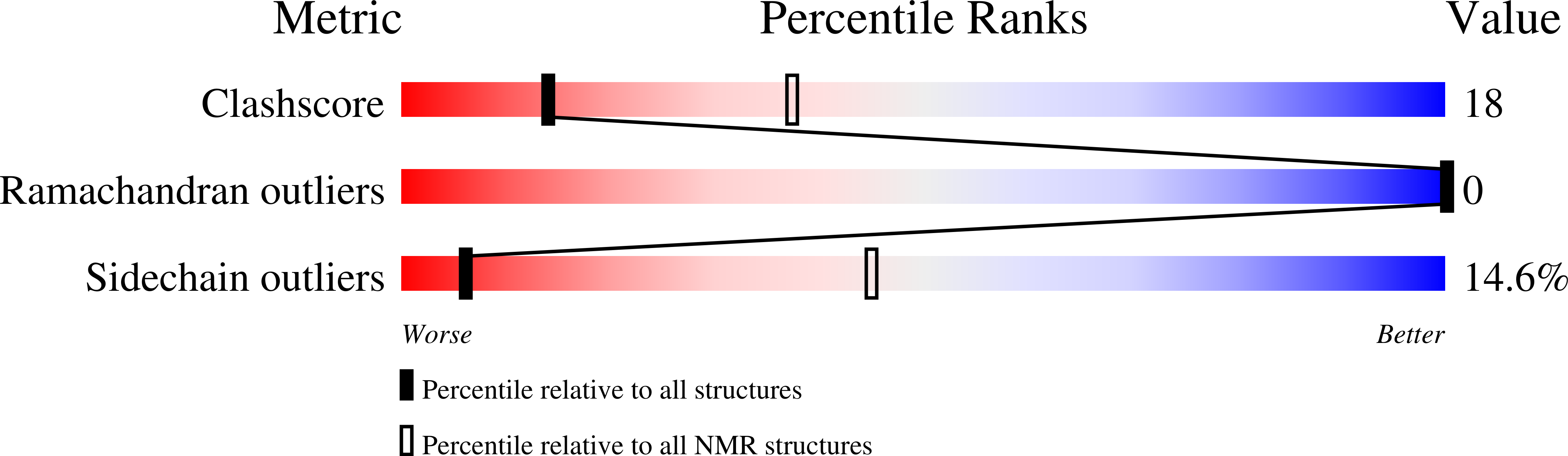Structural Requirements for Cooperativity in Ileal Bile Acid-binding Proteins.
Zanzoni, S., Assfalg, M., Giorgetti, A., D'Onofrio, M., Molinari, H.(2011) J Biol Chem 286: 39307-39317
- PubMed: 21917914
- DOI: https://doi.org/10.1074/jbc.M111.261099
- Primary Citation of Related Structures:
2LBA - PubMed Abstract:
Ileal bile acid-binding proteins (I-BABP), belonging to the family of intracellular lipid-binding proteins, control bile acid trafficking in enterocytes and participate in regulating the homeostasis of these cholesterol-derived metabolites. I-BABP orthologues share the same structural fold and are able to host up to two ligands in their large internal cavities. However variations in the primary sequences determine differences in binding properties such as the degree of binding cooperativity. To investigate the molecular requirements for cooperativity we adopted a gain-of-function approach, exploring the possibility to turn the noncooperative chicken I-BABP (cI-BABP) into a cooperative mutant protein. To this aim we first solved the solution structure of cI-BABP in complex with two molecules of the physiological ligand glycochenodeoxycholate. A comparative structural analysis with closely related members of the same protein family provided the basis to design a double mutant (H99Q/A101S cI-BABP) capable of establishing a cooperative binding mechanism. Molecular dynamics simulation studies of the wild type and mutant complexes and essential dynamics analysis of the trajectories supported the role of the identified amino acid residues as hot spot mediators of communication between binding sites. The emerging picture is consistent with a binding mechanism that can be described as an extended conformational selection model.
Organizational Affiliation:
Department of Biotechnology, University of Verona, 37134 Verona, Italy.