The Mutations Lys 114 --> Gln and Asp 126 --> Asn Disrupt an Intersubunit Salt Bridge and Convert Listeria Innocua Dps Into its Natural Mutant Listeria Monocytogenes Dps. Effects on Protein Stability at Low Ph.
Bellapadrona, G., Chiaraluce, R., Consalvi, V., Ilari, A., Stefanini, S., Chiancone, E.(2007) Proteins 66: 975
- PubMed: 17186524
- DOI: https://doi.org/10.1002/prot.21305
- Primary Citation of Related Structures:
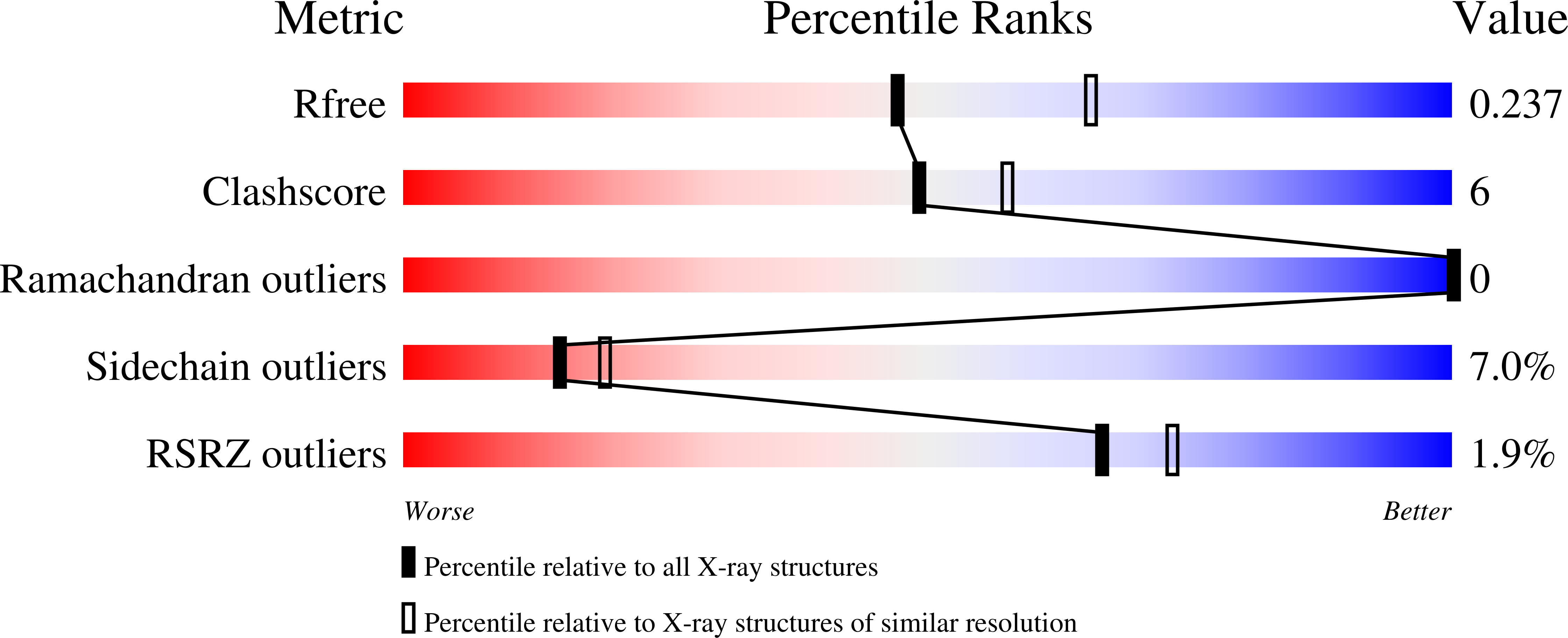
2IY4 - PubMed Abstract:
The stability of the dodecameric Listeria monocytogenes Dps has been compared with that of the Listeria innocua protein. The two proteins differ only in two amino acid residues that form an intersubunit salt-bridge in L. innocua Dps. This salt-bridge is replaced by a hydrogen bonding network in L. monocytogenes Dps as revealed by the X-ray crystal structure. The resistance to low pH and high temperature was assayed for both Dps proteins under equilibrium conditions and kinetically. Despite the identical equilibrium behavior, significant differences in the kinetic stability and activation energy of the unfolding process are apparent at pH 1.5. The higher stability of L. monocytogenes Dps has been accounted for in terms of the persistence of the hydrogen bonding network at this low pH value. In contrast, the salt-bridge between Lys 114 and Asp 126 characteristic of L. innocua Dps is most likely abolished due to protonation of Asp 126.
Organizational Affiliation:
Dipartimento di Scienze Biochimiche A. Rossi Fanelli, Università La Sapienza, Rome, Italy.