A Novel Conotoxin Inhibitor of Kv1.6 Channel and nAChR Subtypes Defines a New Superfamily of Conotoxins
Imperial, J.S., Bansal, P.S., Alewood, P.F., Daly, N.L., Craik, D.J., Sporning, A., Terlau, H., Lopez-Vera, E., Bandyopadhyay, P.K., Olivera, B.M.(2006) Biochemistry 45: 8331-8340
- PubMed: 16819832
- DOI: https://doi.org/10.1021/bi060263r
- Primary Citation of Related Structures:
2FQC - PubMed Abstract:
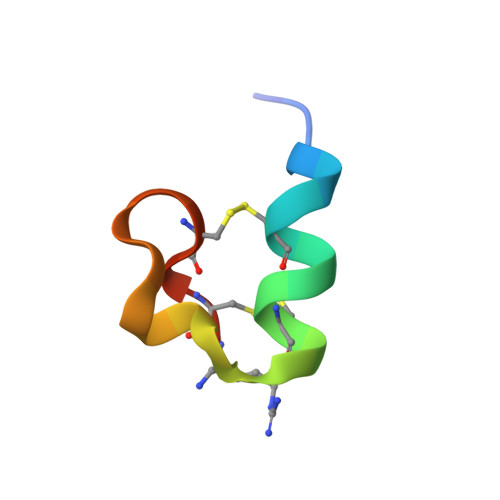
Using assay-directed fractionation of the venom from the vermivorous cone snail Conus planorbis, we isolated a new conotoxin, designated pl14a, with potent activity at both nicotinic acetylcholine receptors and a voltage-gated potassium channel subtype. pl14a contains 25 amino acid residues with an amidated C-terminus, an elongated N-terminal tail (six residues), and two disulfide bonds (1-3, 2-4 connectivity) in a novel framework distinct from other conotoxins. The peptide was chemically synthesized, and its three-dimensional structure was demonstrated to be well-defined, with an alpha-helix and two 3(10)-helices present. Analysis of a cDNA clone encoding the prepropeptide precursor of pl14a revealed a novel signal sequence, indicating that pl14a belongs to a new gene superfamily, the J-conotoxin superfamily. Five additional peptides in the J-superfamily were identified. Intracranial injection of pl14a in mice elicited excitatory symptoms that included shaking, rapid circling, barrel rolling, and seizures. Using the oocyte heterologous expression system, pl14a was shown to inhibit both a K+ channel subtype (Kv1.6, IC50 = 1.59 microM) and neuronal (IC50 = 8.7 microM for alpha3beta4) and neuromuscular (IC50 = 0.54 microM for alpha1beta1 epsilondelta) subtypes of the nicotinic acetylcholine receptor (nAChR). Similarities in sequence and structure are apparent between the middle loop of pl14a and the second loop of a number of alpha-conotoxins. This is the first conotoxin shown to affect the activity of both voltage-gated and ligand-gated ion channels.
Organizational Affiliation:
Department of Biology, University of Utah, Salt Lake City, Utah 84112, USA. imperial@biology.utah.edu