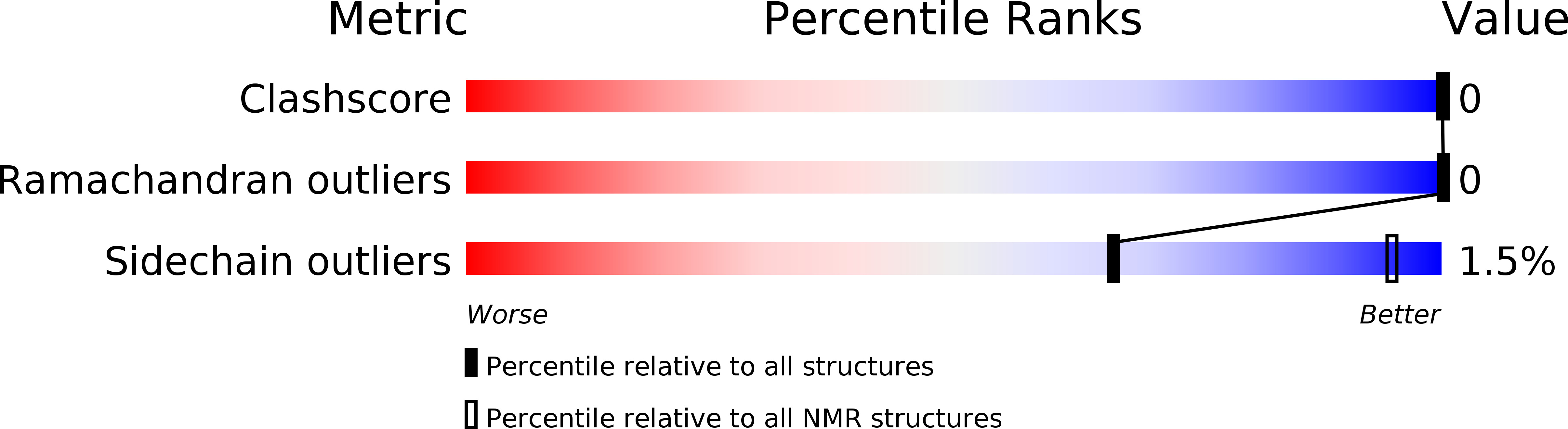A Tensor-Free Method for the Structural and Dynamical Refinement of Proteins using Residual Dipolar Couplings.
Camilloni, C., Vendruscolo, M.(2015) J Phys Chem B 119: 653-661
- PubMed: 24824082
- DOI: https://doi.org/10.1021/jp5021824
- Primary Citation of Related Structures:
2MOR - PubMed Abstract:
Residual dipolar couplings (RDCs) are parameters measured in nuclear magnetic resonance spectroscopy that can provide exquisitely detailed information about the structure and dynamics of biological macromolecules. We describe here a method of using RDCs for the structural and dynamical refinement of proteins that is based on the observation that the RDC between two atomic nuclei depends directly on the angle ϑ between the internuclear vector and the external magnetic field. For every pair of nuclei for which an RDC is available experimentally, we introduce a structural restraint to minimize the deviation from the value of the angle ϑ derived from the measured RDC and that calculated in the refinement protocol. As each restraint involves only the calculation of the angle ϑ of the corresponding internuclear vector, the method does not require the definition of an overall alignment tensor to describe the preferred orientation of the protein with respect to the alignment medium. Application to the case of ubiquitin demonstrates that this method enables an accurate refinement of the structure and dynamics of this protein to be obtained.
Organizational Affiliation:
Department of Chemistry, University of Cambridge , Cambridge CB2 1EW, U.K.