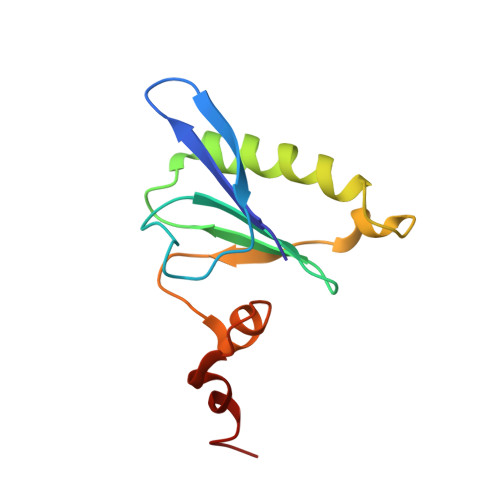
Gelsolin-like activation of villin: calcium sensitivity of the long helix in domain 6.
Fedechkin, S.O., Brockerman, J., Pfaff, D.A., Burns, L., Webb, T., Nelson, A., Zhang, F., Sabantsev, A.V., Melnikov, A.S., McKnight, C.J., Smirnov, S.L.(2013) Biochemistry 52: 7890-7900
- PubMed: 24070253
- DOI: https://doi.org/10.1021/bi400699s
- Primary Citation of Related Structures:
2LLF - PubMed Abstract:
Villin is a gelsolin-like cytoskeleton regulator localized in the brush border at the apical end of epithelial cells. Villin regulates microvilli by bundling F-actin at low calcium levels and severing it at high calcium levels. The villin polypeptide consists of six gelsolin-like repeats (V1-V6) and the unique, actin binding C-terminal headpiece domain (HP). Villin modular fragment V6-HP requires calcium to stay monomeric and bundle F-actin. Our data show that isolated V6 is monomeric and does not bind F-actin at any level of calcium. We propose that the 40-residue unfolded V6-to-HP linker can be a key regulatory element in villin's functions such as its interactions with F-actin. Here we report a calcium-bound solution nuclear magnetic resonance (NMR) structure of V6, which has a gelsolin-like fold with the long α-helix in the extended conformation. Intrinsic tryptophan fluorescence quenching reveals two-Kd calcium binding in V6 (Kd1 of 22 μM and Kd2 of 2.8 mM). According to our NMR data, the conformation of V6 responds the most to micromolar calcium. We show that the long α-helix and the adjacent residues form the calcium-sensitive elements in V6. These observations are consistent with the calcium activation of F-actin severing by villin analogous to the gelsolin helix-straightening mechanism.
Organizational Affiliation:
Department of Chemistry, Western Washington University , 516 High Street, Bellingham, Washington 98225-9150, United States.