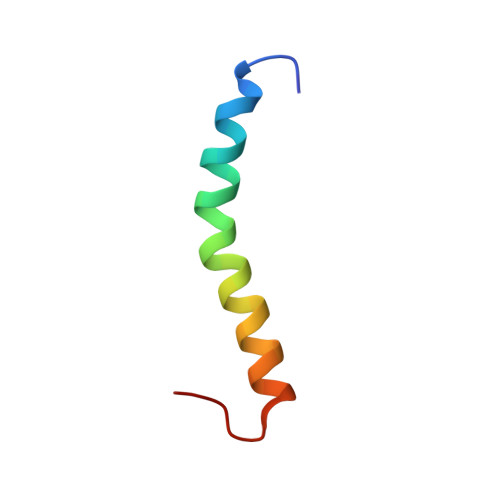
Solution structure of a human minimembrane protein Ost4, a subunit of the oligosaccharyltransferase complex.
Gayen, S., Kang, C.(2011) Biochem Biophys Res Commun 409: 572-576
- PubMed: 21609714
- DOI: https://doi.org/10.1016/j.bbrc.2011.05.050
- Primary Citation of Related Structures:
2LAT - PubMed Abstract:
Oligosaccharyltransferase (OST) is a membrane associated enzyme complex that mediates transfer of an oligosaccharide onto asparagine residue of a protein. Human Ost4 is a small membrane protein and belongs to one of the seven subunits of human OST. This study determined the solution structure of human Ost4 in solvent system using NMR spectroscopy. Ost4 was demonstrated that the residues 5-30 adopt an α-helical structure. A kink structure was observed in the transmembrane domain, which may be important for its function.
Organizational Affiliation:
Experimental Therapeutics Center, The Agency for Science, Technology and Research, Singapore 138669, Singapore.