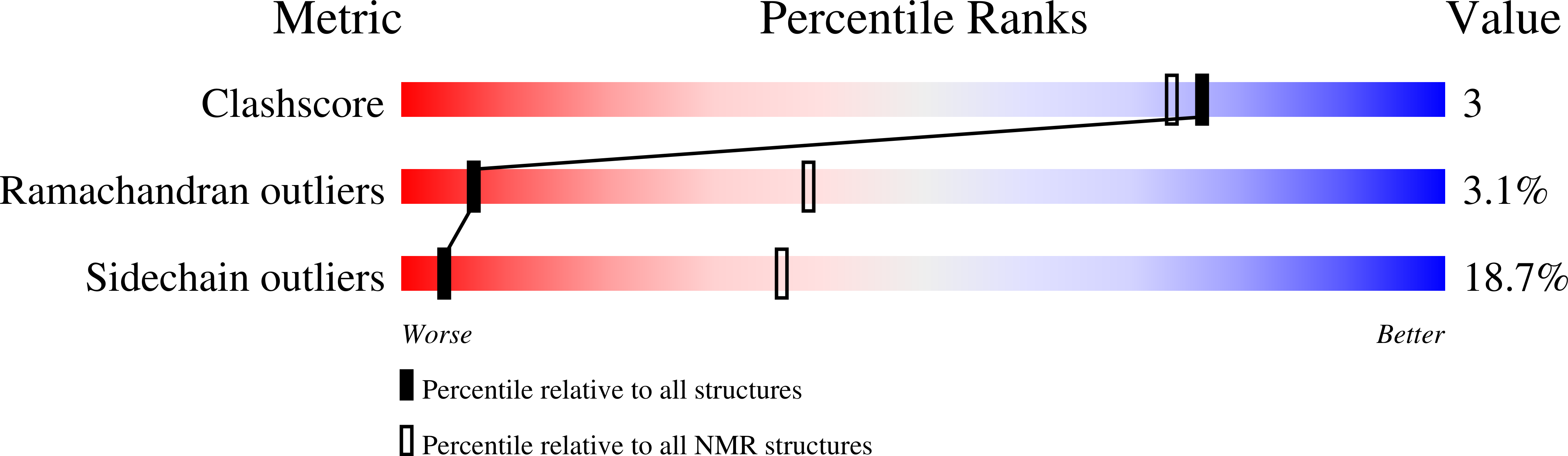Conformational dynamics of the anthrax lethal factor catalytic center.
Dalkas, G.A., Chasapis, C.T., Gkazonis, P.V., Bentrop, D., Spyroulias, G.A.(2010) Biochemistry 49: 10767-10769
- PubMed: 21121613
- DOI: https://doi.org/10.1021/bi1017792
- Primary Citation of Related Structures:
2L0R - PubMed Abstract:
Anthrax lethal factor (LF) is a zinc-metalloprotease that together with the protective antigen constitutes anthrax lethal toxin, which is the most prominent virulence factor of the anthrax disease. The solution nuclear magnetic resonance and in silico conformational dynamics of the 105 C-terminal residues of the LF catalytic core domain in its apo form are described here. The polypeptide adopts a compact structure even in the absence of the Zn(2+) cofactor, while the 40 N-terminal residues comprising the metal ligands and residues that participate in substrate and inhibitor recognition exhibit more flexibility than the C-terminal region.
Organizational Affiliation:
Department of Pharmacy, University of Patras, GR-26504 Patras, Greece.