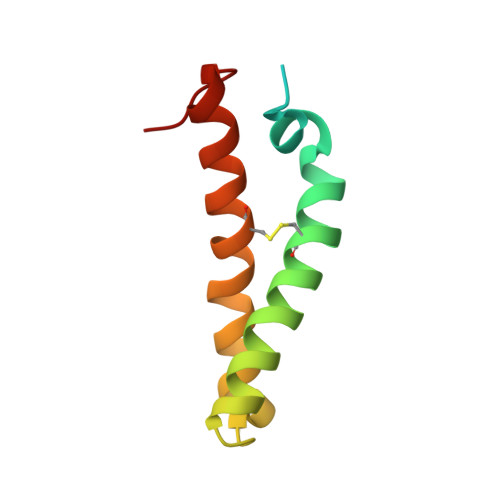
Prion fibrillization is mediated by a native structural element that comprises helices H2 and H3.
Adrover, M., Pauwels, K., Prigent, S., de Chiara, C., Xu, Z., Chapuis, C., Pastore, A., Rezaei, H.(2010) J Biol Chem 285: 21004-21012
- PubMed: 20375014
- DOI: https://doi.org/10.1074/jbc.M110.111815
- Primary Citation of Related Structures:
2KTM - PubMed Abstract:
Aggregation and misfolding of the prion protein (PrP) are thought to be the cause of a family of lethal neurodegenerative diseases affecting humans and other animals. Although the structures of PrP from several species have been solved, still little is known about the mechanisms that lead to the misfolded species. Here, we show that the region of PrP comprising the hairpin formed by the helices H2 and H3 is a stable independently folded unit able to retain its secondary and tertiary structure also in the absence of the rest of the sequence. We also prove that the isolated H2H3 is highly fibrillogenic and forms amyloid fibers morphologically similar to those obtained for the full-length protein. Fibrillization of H2H3 but not of full-length PrP is concomitant with formation of aggregates. These observations suggest a "banana-peeling" mechanism for misfolding of PrP in which H2H3 is the aggregation seed that needs to be first exposed to promote conversion from a helical to a beta-rich structure.
Organizational Affiliation:
MRC National Institute for Medical Research, The Ridgeway, London NW7 1AA, United Kingdom.