Assignment of the Ferriheme Resonances of the Low-Spin Complexes of Nitrophorins 1 and 4 by (1)H and (13)C NMR Spectroscopy: Comparison to Structural Data Obtained from X-ray Crystallography.
Shokhireva, T.Kh., Weichsel, A., Smith, K.M., Berry, R.E., Shokhirev, N.V., Balfour, C.A., Zhang, H., Montfort, W.R., Walker, F.A.(2007) Inorg Chem 46: 2041-2056
- PubMed: 17290983
- DOI: https://doi.org/10.1021/ic061408l
- Primary Citation of Related Structures:
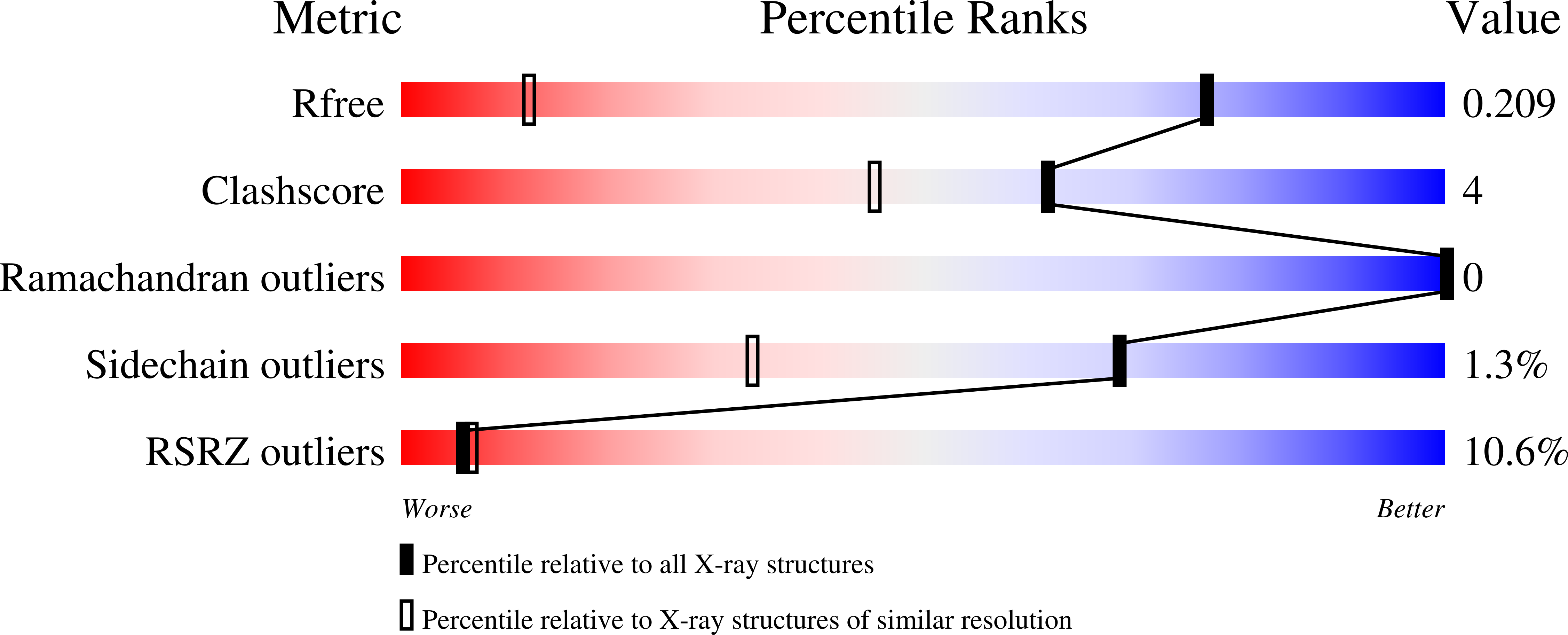
2HYS - PubMed Abstract:
In this work we report the assignment of the majority of the ferriheme resonances of low-spin nitrophorins (NP) 1 and 4 and compare them to those of NP2, published previously. It is found that the structure of the ferriheme complexes of NP1 and NP4, in terms of the orientation of the ligand(s), can be determined with good accuracy by NMR techniques in the low-spin forms and that angle plots proposed previously (Shokhirev, N. V.; Walker, F. A. J. Biol. Inorg. Chem. 1998, 3, 581-594) describe the angle of the effective nodal plane of the axial ligands in solution. The effective nodal plane of low-spin NP1, NP4, and NP2 complexes is in all cases of imidazole and histamine complexes quite similar to the average of the His-59 or -57 and the exogenous ligand angles seen in the X-ray crystal structures. For the cyanide complexes of the nitrophorins, however, the effective nodal plane of the axial ligand does not coincide with the actual histidine-imidazole plane orientation. This appears to be a result of the contribution of an additional source of asymmetry, the orientation of one of the zero-ruffling lines of the heme. Probably this effect exists for the imidazole and histamine complexes as well, but because the effect of asymmetry that occurs from planar exogenous axial ligands is much larger than the effect of heme ruffling the effect of the zero-ruffling line can only be detected for the cyanide complexes, where the only ligand plane is that of the proximal histidine. The three-dimensional structures of the three NP-CN complexes, including that of NP2-CN reported herein, confirm the high degree of ruffling of these complexes. There is an equilibrium between the two heme orientations (A and B) that depends on the heme cavity shape and changes somewhat with exogenous axial ligand. The A:B ratio can be much more accurately measured by NMR spectroscopy than by X-ray crystallography.
Organizational Affiliation:
Department of Chemistry and Biochemistry, The University of Arizona, Tucson, Arizona 85721, USA.