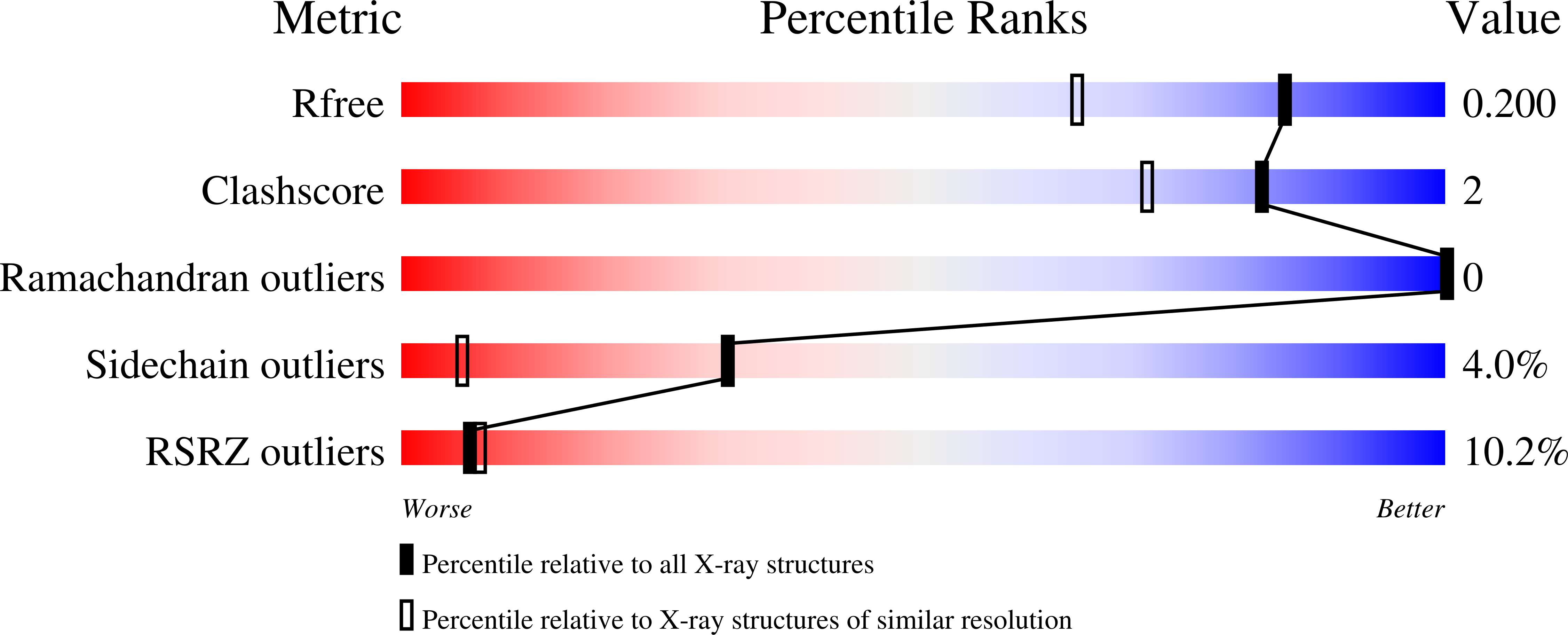
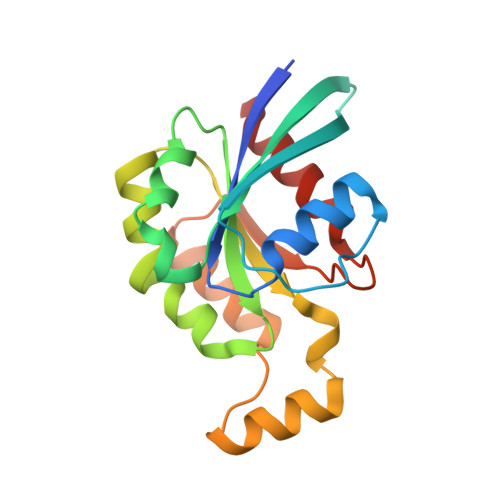
Structure of a constitutively activated RhoA mutant (Q63L) at 1.55 A resolution.
Longenecker, K., Read, P., Lin, S.K., Somlyo, A.P., Nakamoto, R.K., Derewenda, Z.S.(2003) Acta Crystallogr D Biol Crystallogr 59: 876-880
- PubMed: 12777804
- DOI: https://doi.org/10.1107/s0907444903005390
- Primary Citation of Related Structures:
1KMQ - PubMed Abstract:
Mutants of the small G protein RhoA that are deficient in GTPase activity and thereby exhibit constitutive molecular signaling activity are commonly used to discover its cellular functions. In particular, two such mutants, Gly14-->Val (G14V) and Gln63-->Leu (Q63L), are often used interchangeably for such studies. However, while their in vitro rates of GTP hydrolysis are very similar, differences are observed in their other functional properties. The structure of G14V-RhoA is known; in order to assess whether structural variations are responsible for functional differences, the crystal structure of a Q63L-RhoA bound to the GTP-analog 5'-guanylylimidodiphosphate (GMPPNP) was determined at 1.5 A resolution. Overall, the structure is very similar to that of G14V-RhoA, but the significantly higher resolution data permit an improved basis for structural analysis and comparison. The data support the notion that differences observed between the mutants in vivo are likely to arise from altered affinities for RhoGDI and not from direct structural differences.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, VA 22908, USA.