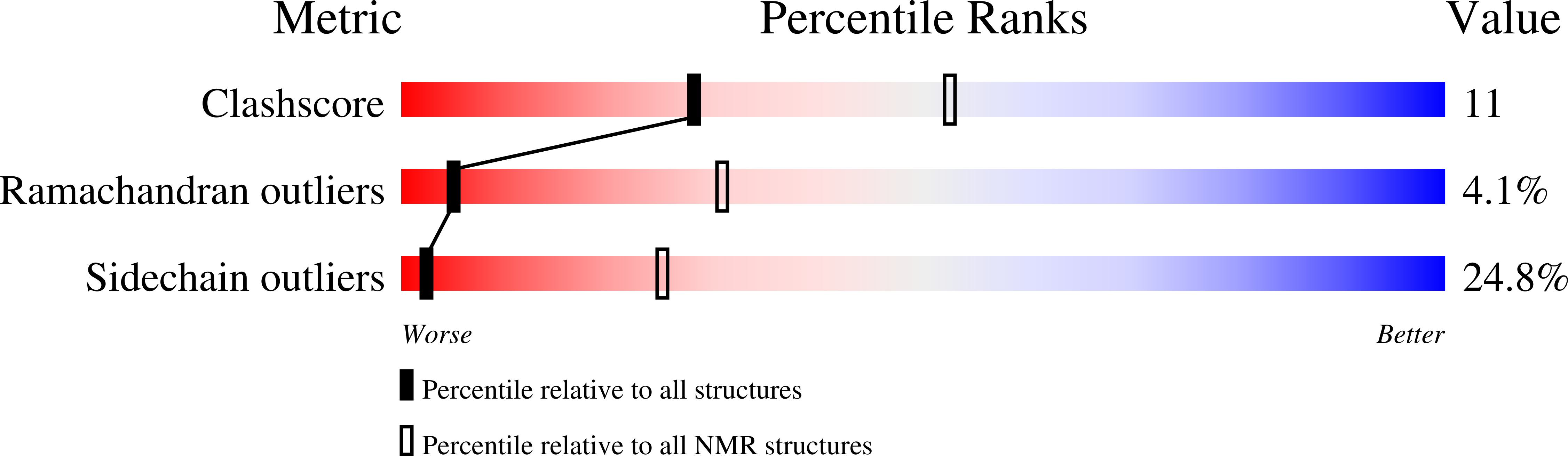Solution structure of human peptidyl prolyl isomerase like protein 1 and insights into its interaction with SKIP
Xu, C., Zhang, J., Huang, X., Sun, J., Xu, Y., Tang, Y., Wu, J., Shi, Y., Huang, Q., Zhang, Q.(2006) J Biol Chem 281: 15900-15908
- PubMed: 16595688
- DOI: https://doi.org/10.1074/jbc.M511155200
- Primary Citation of Related Structures:
1XWN - PubMed Abstract:
The human PPIL1 (peptidyl prolyl isomerase-like protein 1) is a specific component of human 35 S U5 small nuclear ribonucleoprotein particle and 45 S activated spliceosome. It is recruited by SKIP, another essential component of 45 S activated spliceosome, into spliceosome just before the catalytic step 1. It stably associates with SKIP, which also exists in 35 S and activated spliceosome as a nuclear matrix protein. We report here the solution structure of PPIL1 determined by NMR spectroscopy. The structure of PPIL1 resembles other members of the cyclophilin family and exhibits PPIase activity. To investigate its interaction with SKIP in vitro, we identified the SKIP contact region by GST pulldown experiments and surface plasmon resonance. We provide direct evidence of PPIL1 stably associated with SKIP. The dissociation constant is 1.25 x 10(-7) M for the N-terminal peptide of SKIP-(59-129) with PPIL1. We also used chemical shift perturbation experiments to show the possible SKIP binding interface on PPIL1. These results illustrated that a novel cyclophilin-protein contact mode exists in the PPIL1-SKIP complex during activation of the spliceosome. The biological implication of this binding with spliceosome rearrangement during activation is discussed.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, 230026, China.