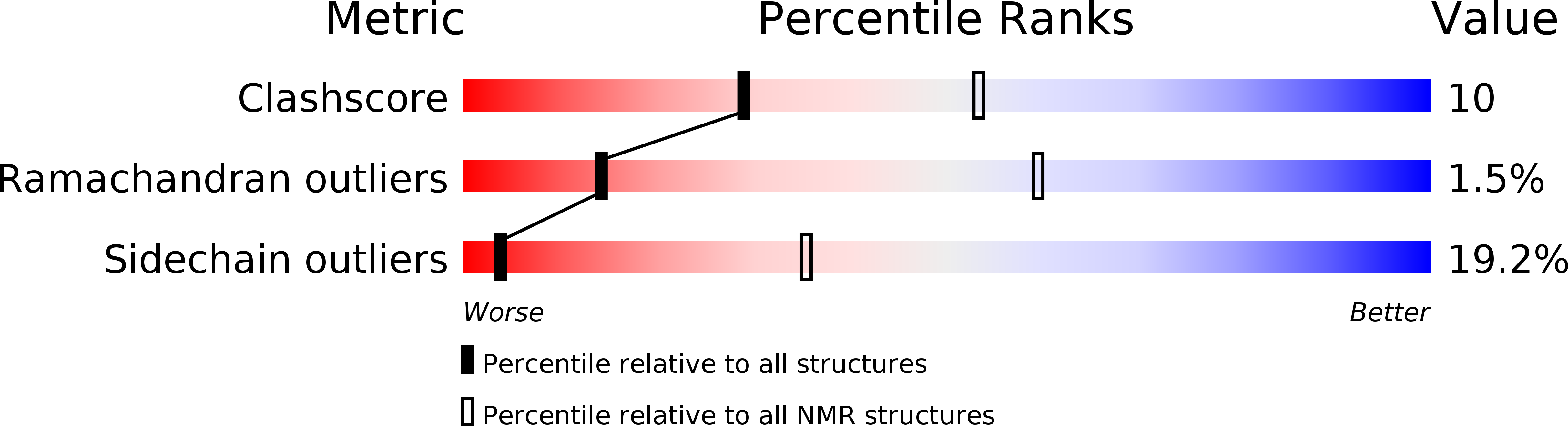Solution structure of turkey ovomucoid third domain as determined from nuclear magnetic resonance data.
Krezel, A.M., Darba, P., Robertson, A.D., Fejzo, J., Macura, S., Markley, J.L.(1994) J Mol Biol 242: 203-214
- PubMed: 8089842
- DOI: https://doi.org/10.1006/jmbi.1994.1573
- Primary Citation of Related Structures:
1TUR - PubMed Abstract:
The solution structure of the 56 amino acid residue turkey ovomucoid third domain was determined by n.m.r. methods. Of the 661 distance constraints used in the calculations, 120 were determined by quadratic approximation of the cross-relaxation rates. The remaining constraints were crudely estimated from a more standard analysis of NOESY spectra. Additionally, 29 torsion angle constraints, 17 hydrogen bonds, and three disulfide bridges were used in the structure calculations. Stereospecific assignments were accomplished for 24 beta-methylene groups and six isopropyl methyl groups (43% chiral assignments). The addition of more accurate distance constraints to the distance geometry/simulated annealing approach resulted in a significant reduction in the dispersion of calculated backbone torsion angles and root-mean-square deviations between structures. Detailed comparisons have been made between the n.m.r. structures of OMTKY3 and published X-ray structures of the same protein and of closely related avian ovomucoid third domains. The refinement with more accurate distance constraints reduced differences between families of the n.m.r. and the X-ray structures.
Organizational Affiliation:
Department of Biochemistry, College of Agricultural and Life Sciences, University of Wisconsin-Madison 53706.