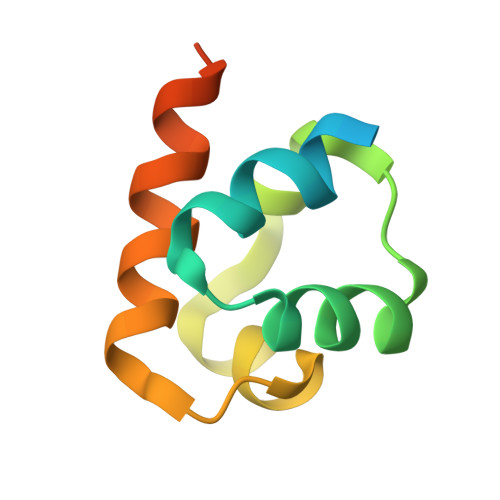
The solution structure of the S.cerevisiae Ste11 MAPKKK SAM domain and its partnership with Ste50.
Kwan, J.J., Warner, N., Pawson, T., Donaldson, L.W.(2004) J Mol Biol 342: 681-693
- PubMed: 15327964
- DOI: https://doi.org/10.1016/j.jmb.2004.06.064
- Primary Citation of Related Structures:
1OW5 - PubMed Abstract:
Ste11 is a MAPKKK from Saccharomyces cerevisiae that helps mediate the response to mating pheromone and the ability to thrive in high-salt environments. These diverse functions are facilitated by a direct interaction between the SAM domain of Ste11 with the SAM domain of its regulatory partner, Ste50. We have solved the NMR structure of the Ste11 SAM domain (PDB 1OW5), which reveals a compact, five alpha-helix bundle and a high degree of structural similarity to the Polyhomeotic SAM domain. The combined study of Ste11 SAM rotational correlation times and crosslinking to Ste50-SAM has suggested a mode through which Ste11-SAM oligomerizes and selectively associates with Ste50-SAM. To probe homotypic and heterotypic interations, Ste11-SAM variants each containing a substitution of a surface-exposed hydrophobic residue were constructed. An I59R variant of Ste11-SAM, disrupted binding to Ste50-SAM in vitro. Yeast expressing full-length Ste11-I59R could neither respond to mating pheromone nor thrive in high salt media-demonstrating that the interaction between Ste11 and Ste50 SAM domains is a prerequisite for key signal transduction events.
Organizational Affiliation:
Department of Biology, York University, 4700 Keele Street, Toronto, Ontario, Canada M3J 1P3.