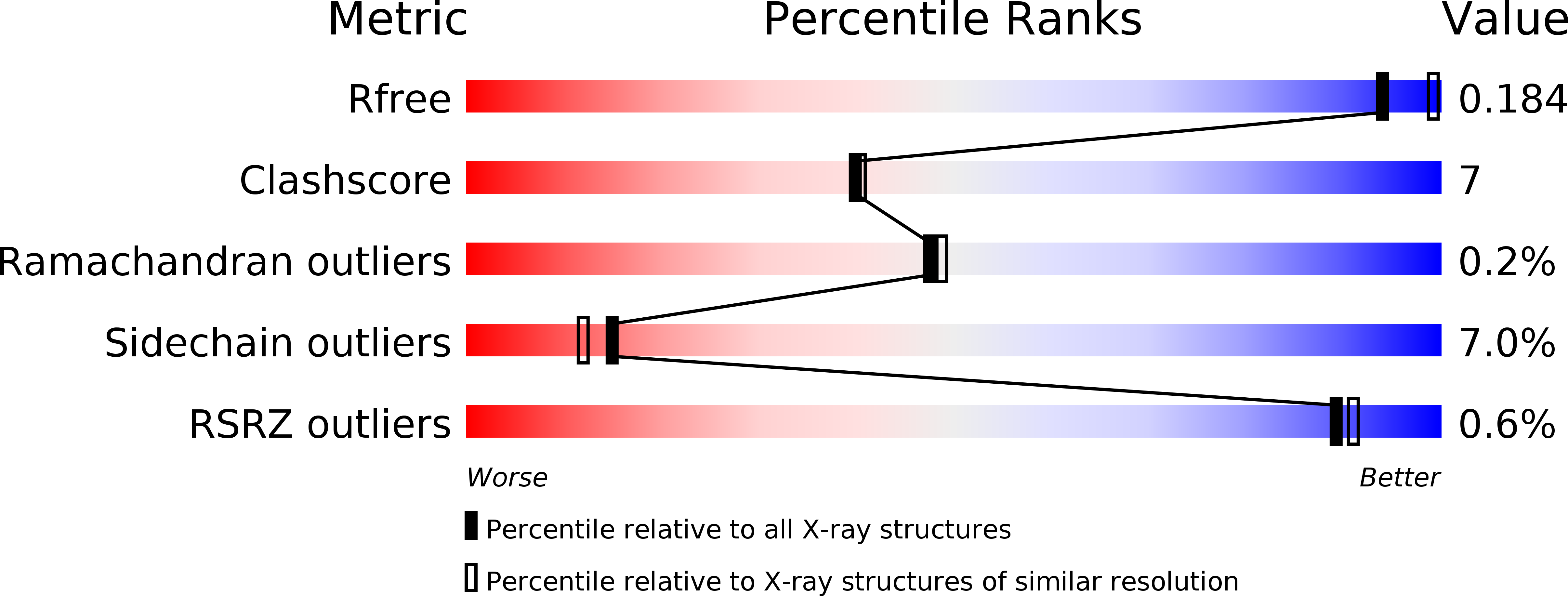
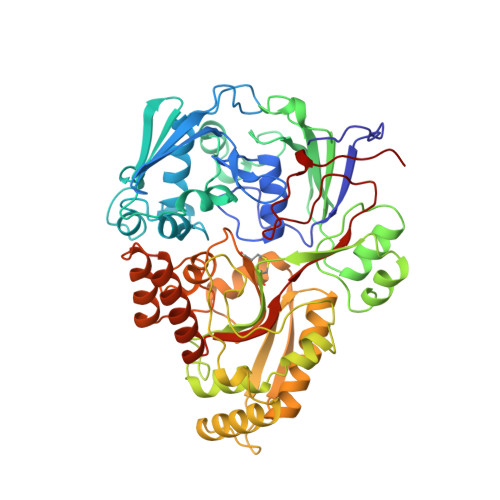
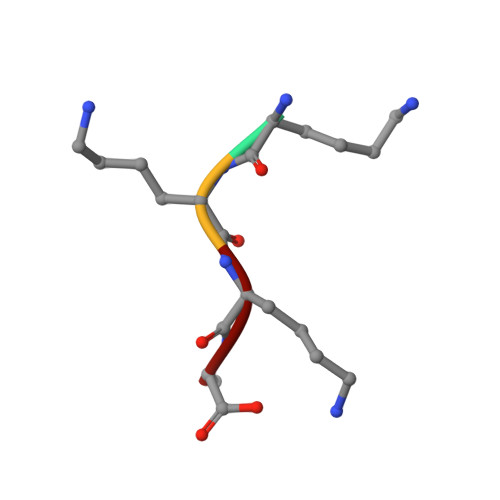
The crystal structures of the oligopeptide-binding protein OppA complexed with tripeptide and tetrapeptide ligands.
Tame, J.R., Dodson, E.J., Murshudov, G., Higgins, C.F., Wilkinson, A.J.(1995) Structure 3: 1395-1406
- PubMed: 8747465
- DOI: https://doi.org/10.1016/s0969-2126(01)00276-3
- Primary Citation of Related Structures:
1OLC, 2OLB - PubMed Abstract:
The periplasmic oligopeptide-binding protein OppA has a remarkably broad substrate specificity, binding peptides of two or five amino-acid residues with high affinity, but little regard to sequence. It is therefore an ideal system for studying how different chemical groups can be accommodated in a protein interior. The ability of the protein to bind peptides of different lengths has been studied by co-crystallising it with different ligands.
Organizational Affiliation:
Department of Chemistry, University of York, UK.