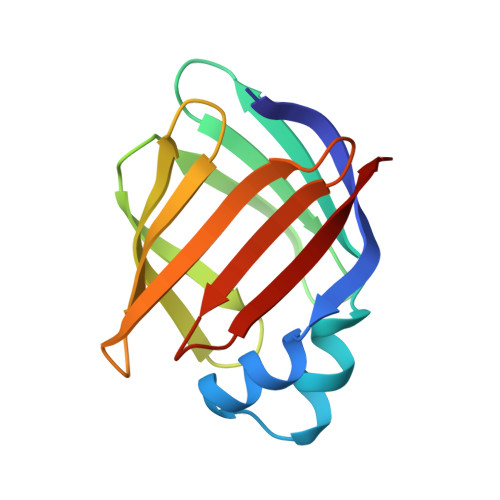
Solution structure of human intestinal fatty acid binding protein with a naturally-occurring single amino acid substitution (A54T) that is associated with altered lipid metabolism
Zhang, F., Luecke, C., Baier, L.J., Sacchettini, J.C., Hamilton, J.A.(2003) Biochemistry 42: 7339-7347
- PubMed: 12809489
- DOI: https://doi.org/10.1021/bi0273617
- Primary Citation of Related Structures:
1KZW, 1KZX - PubMed Abstract:
The human intestinal fatty acid binding protein (I-FABP) belongs to a family of intracellular lipid binding proteins. This 15 kDa protein binds dietary long-chain fatty acids in the cytosol of enterocytes. A naturally-occurring nucleotide polymorphism at codon 54, which produces either an alanine-containing (A54) or a threonine-containing (T54) protein, has been identified. These two I-FABP forms display differential binding and transport of fatty acids across cells, and their alleles are associated with in vivo insulin resistance and/or altered lipid metabolism in several human populations. The three-dimensional solution structure of the more common A54 form was previously determined in our lab. A direct comparison between human A54 and T54 I-FABP has now been performed to help elucidate the structural origins of their physiological distinctions. The solution structure of T54 I-FABP is highly homologous to that of A54 I-FABP, with the same overall three-dimensional fold that includes an antiparallel beta-clam motif. Chemical shift differences between the two proteins suggest only minor local structural changes within the "portal region" and no significant alterations elsewhere. Hence, the slightly stronger binding of fatty acids to T54 I-FABP does not originate from residues in direct contact with the bound fatty acid. Instead, it appears that the larger Thr(54) side chain affects the passage of the ligand through the entry portal. Structural details of this portal region will be discussed in view of the influence residue 54 exerts on the functional properties of human I-FABP.
Organizational Affiliation:
Department of Physiology and Biophysics, Boston University School of Medicine, Boston, Massachusetts 02118-2526, USA.