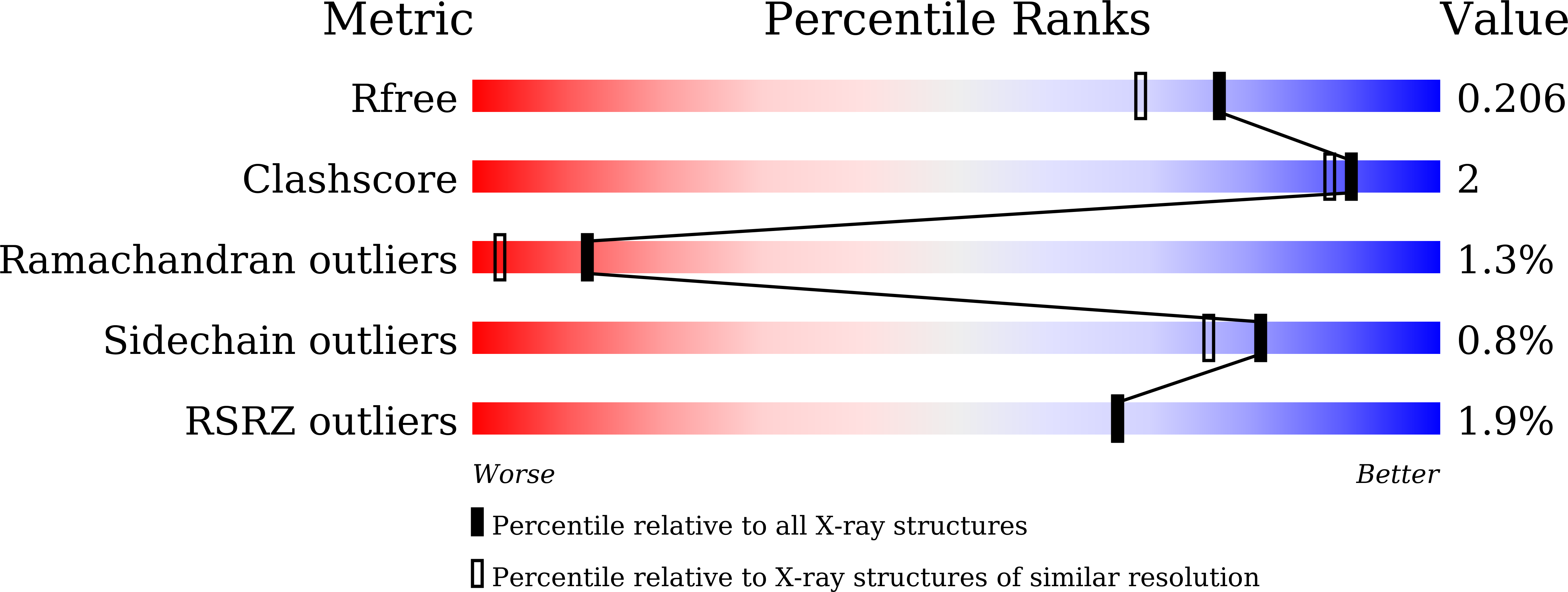
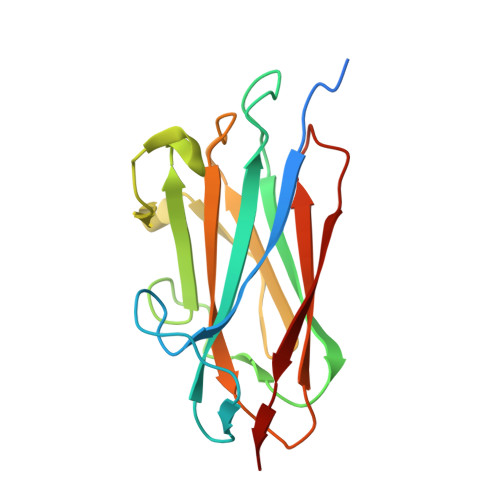
Structure of the hypothetical protein TTHA1873 from Thermus thermophilus.
Yuvaraj, I., Chaudhary, S.K., Jeyakanthan, J., Sekar, K.(2022) Acta Crystallogr F Struct Biol Commun 78: 338-346
- PubMed: 36048084
- DOI: https://doi.org/10.1107/S2053230X22008457
- Primary Citation of Related Structures:
7WRK, 7WWN, 7WWO - PubMed Abstract:
The crystal structure of an uncharacterized hypothetical protein, TTHA1873 from Thermus thermophilus, has been determined by X-ray crystallography to a resolution of 1.78 Å using the single-wavelength anomalous dispersion method. The protein crystallized as a dimer in two space groups: P4 3 2 1 2 and P6 1 22. Structural analysis of the hypothetical protein revealed that the overall fold of TTHA1873 has a β-sandwich jelly-roll topology with nine β-strands. TTHA1873 is a dimeric metal-binding protein that binds to two Ca 2+ ions per chain, with one on the surface and the other stabilizing the dimeric interface of the two chains. A structural homology search indicates that the protein has moderate structural similarity to one domain of cell-surface proteins or agglutinin receptor proteins. Red blood cells showed visible agglutination at high concentrations of the hypothetical protein.
Organizational Affiliation:
Department of Computational and Data Sciences, Indian Institute of Science, Bangalore 560 012, India.