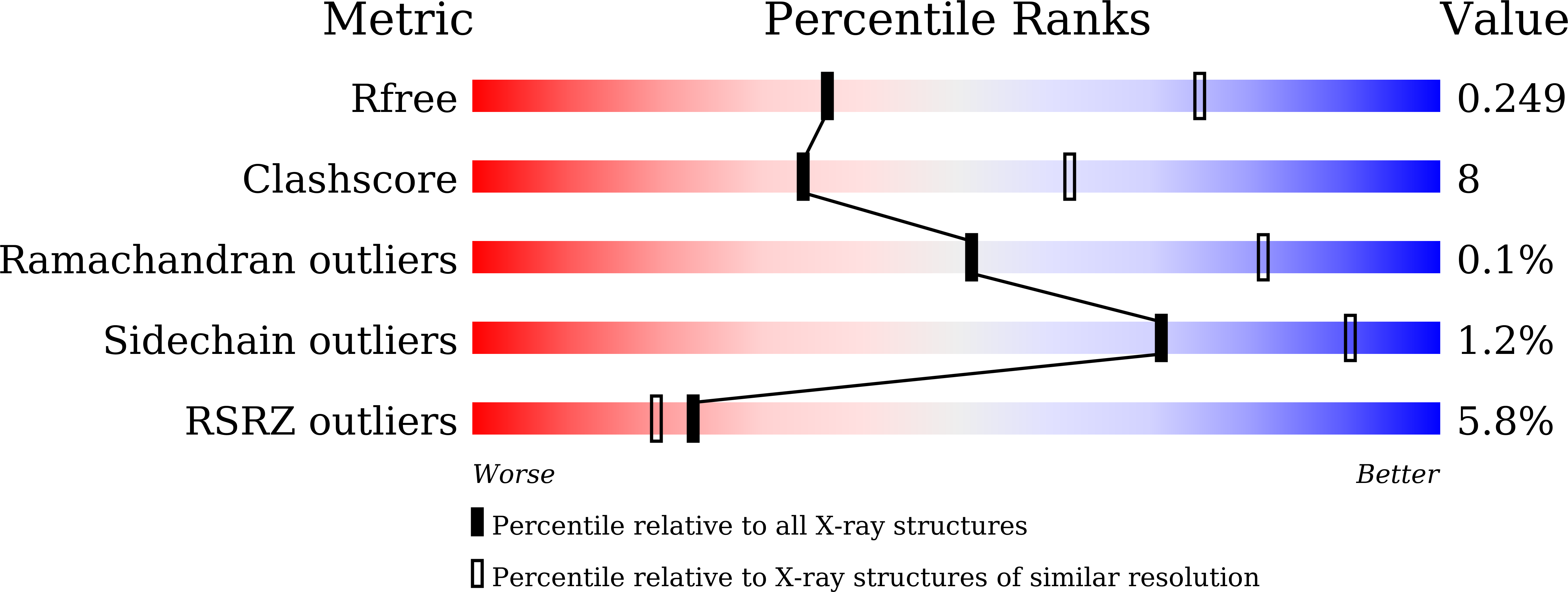
Identification and Structure Analysis of an Unusual Halohydrin Dehalogenase for Highly Chemo-, Regio- and Enantioselective Bio-Nitration of Epoxides.
Wang, H.H., Wan, N.W., Miao, R.P., He, C.L., Chen, Y.Z., Liu, Z.Q., Zheng, Y.G.(2022) Angew Chem Int Ed Engl 61: e202205790-e202205790
- PubMed: 35856897
- DOI: https://doi.org/10.1002/anie.202205790
- Primary Citation of Related Structures:
7WKQ - PubMed Abstract:
We report the discovery of an unusual halohydrin dehalogenase, HHDHamb, that can work under relatively low acidic conditions and extremely low temperatures for the bio-nitration of epoxides using nitrite as a nitrating agent. The bio-nitration strategy exhibits high chemo-, regio-, and enantioselectivity, catalyzing the kinetic resolution of various epoxides to enantiopure β-nitroalcohols with nitro-bearing stereocenters in up to 41 % isolated yield and >99 % enantiomeric excess (ee). Additionally, the bio-nitration method displays a high reaction efficiency and can be performed on a gram scale. We also solved the crystal structure of HHDHamb to understand the possible structural determinants of chemoselectivity control in the bio-nitration reaction.
Organizational Affiliation:
Key Laboratory of Bioorganic Synthesis of Zhejiang Province, College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou, 310014, China.