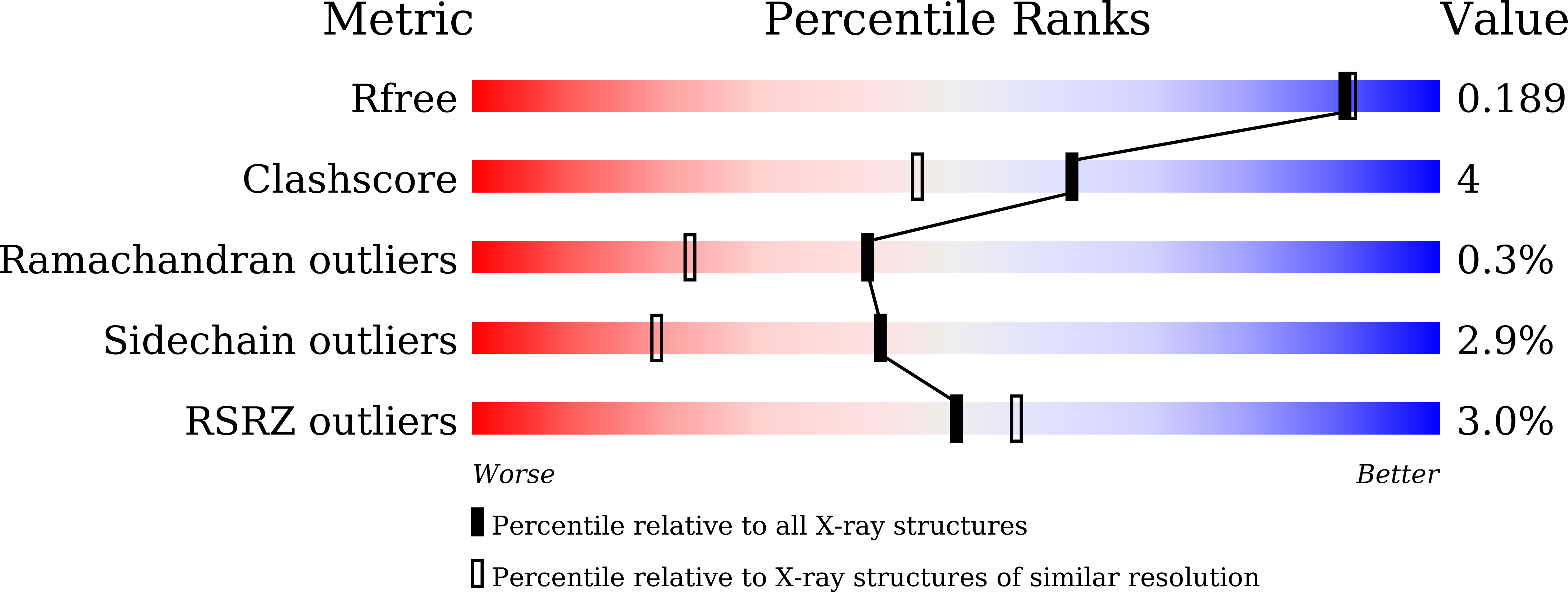
Structural and Functional Insights into a Nonheme Iron- and alpha-Ketoglutarate-Dependent Halogenase That Catalyzes Chlorination of Nucleotide Substrates.
Dai, L., Zhang, X., Hu, Y., Shen, J., Zhang, Q., Zhang, L., Min, J., Chen, C.C., Liu, Y., Huang, J.W., Guo, R.T.(2022) Appl Environ Microbiol 88: e0249721-e0249721
- PubMed: 35435717
- DOI: https://doi.org/10.1128/aem.02497-21
- Primary Citation of Related Structures:
7W5S, 7W5T, 7W5V - PubMed Abstract:
Nonheme iron- and α-ketoglutarate (αKG)-dependent halogenases (NHFeHals), which catalyze the regio- and stereoselective halogenation of the unactivated C( sp 3 )-H bonds, exhibit tremendous potential in the challenging asymmetric halogenation. AdeV from Actinomadura sp. ATCC 39365 is the first identified carrier protein-free NHFeHal that catalyzes the chlorination of nucleotide 2'-deoxyadenosine-5'-monophosphate (2'-dAMP) to afford 2'-chloro-2'-deoxyadenosine-5'-monophosphate. Here, we determined the complex crystal structures of AdeV/Fe II /Cl and AdeV/Fe II /Cl/αKG at resolutions of 1.76 and 1.74 Å, respectively. AdeV possesses a typical β-sandwich topology with H194, H252, αKG, chloride, and one water molecule coordinating Fe II in the active site. Molecular docking, mutagenesis, and biochemical analyses reveal that the hydrophobic interactions and hydrogen bond network between the substrate-binding pocket and the adenine, deoxyribose, and phosphate moieties of 2'-dAMP are essential for substrate recognition. Residues H111, R177, and H192 might play important roles in the second-sphere interactions that control reaction partitioning. This study provides valuable insights into the catalytic selectivity of AdeV and will facilitate the rational engineering of AdeV and other NHFeHals for synthesis of halogenated nucleotides. IMPORTANCE Halogenated nucleotides are a group of important antibiotics and are clinically used as antiviral and anticancer drugs. AdeV is the first carrier protein-independent nonheme iron- and α-ketoglutarate (αKG)-dependent halogenase (NHFeHal) that can selectively halogenate nucleotides and exhibits restricted substrate specificity toward several 2'-dAMP analogues. Here, we determined the complex crystal structures of AdeV/Fe II /Cl and AdeV/Fe II /Cl/αKG. Molecular docking, mutagenesis, and biochemical analyses provide important insights into the catalytic selectivity of AdeV. This study will facilitate the rational engineering of AdeV and other carrier protein-independent NHFeHals for synthesis of halogenated nucleotides.
Organizational Affiliation:
State Key Laboratory of Biocatalysis and Enzyme Engineering, Hubei Hongshan Laboratory, Hubei Collaborative Innovation Center for Green Transformation of Bio-Resources, Hubei Key Laboratory of Industrial Biotechnology, School of Life Sciences, Hubei University, Wuhan, People's Republic of China.