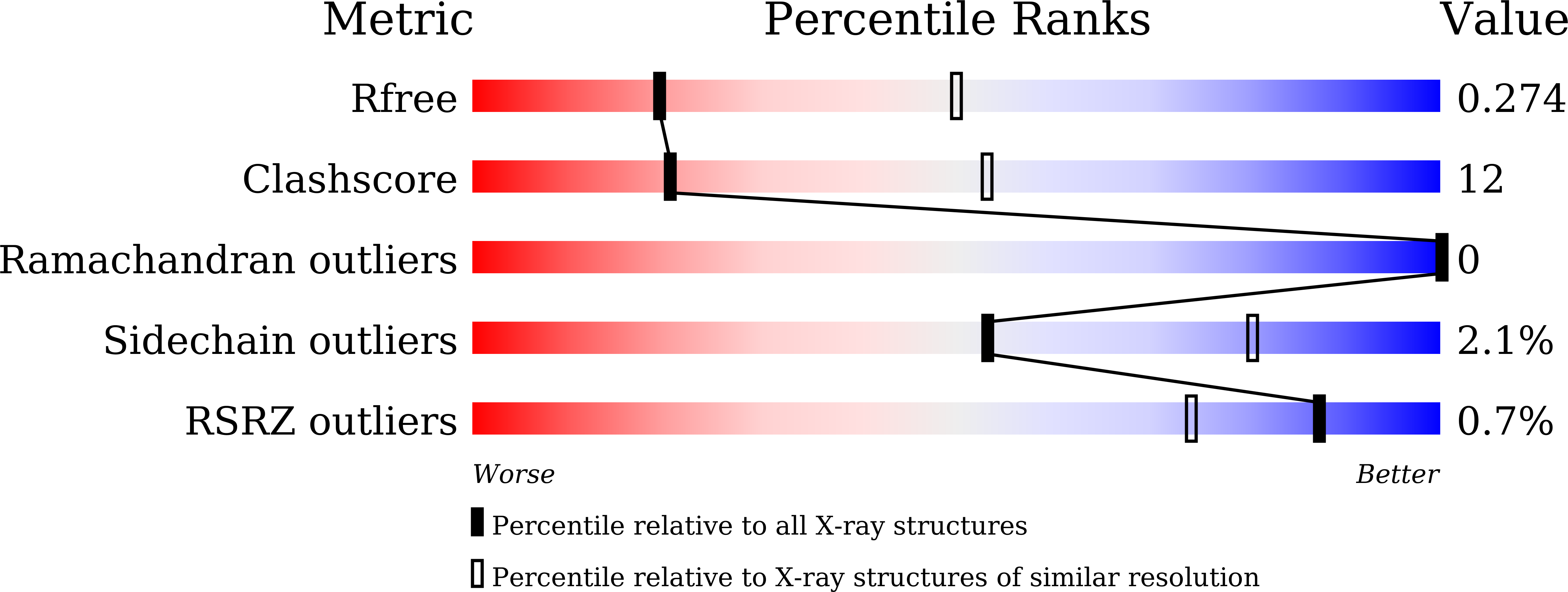
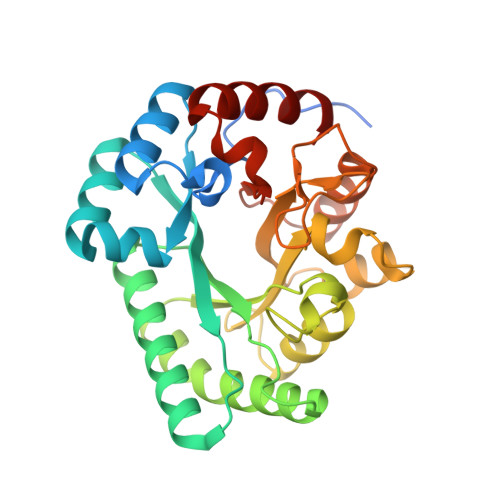
Crystal structure of a novel putative sugar isomerase from the psychrophilic bacterium Paenibacillus sp. R4.
Kwon, S., Ha, H.J., Kang, Y.J., Sung, J.H., Hwang, J., Lee, M.J., Lee, J.H., Park, H.H.(2021) Biochem Biophys Res Commun 585: 48-54
- PubMed: 34784551
- DOI: https://doi.org/10.1016/j.bbrc.2021.11.026
- Primary Citation of Related Structures:
7VPF - PubMed Abstract:
Sugar isomerases (SIs) catalyze the reversible conversion of aldoses to ketoses. A novel putative SI gene has been identified from the genome sequence information on the psychrophilic bacterium Paenibacillus sp. R4. Here, we report the crystal structure of the putative SI from Paenibacillus sp. R4 (PbSI) at 2.98 Å resolution. It was found that the overall structure of PbSI adopts the triose-phosphate isomerase (TIM) barrel fold. PbSI was also identified to have two heterogeneous metal ions as its cofactors at the active site in the TIM barrel, one of which was confirmed as a Zn ion through X-ray anomalous scattering and inductively coupled plasma mass spectrometry analysis. Structural comparison with homologous SI proteins from mesophiles, hyperthermophiles, and a psychrophile revealed that key residues in the active site are well conserved and that dimeric PbSI is devoid of the extended C-terminal region, which tetrameric SIs commonly have. Our results provide novel structural information on the cold-adaptable SI, including information on the metal composition in the active site.
Organizational Affiliation:
Department of Biotechnology, Konkuk University, Chungju, Chungbuk, 27478, Republic of Korea.