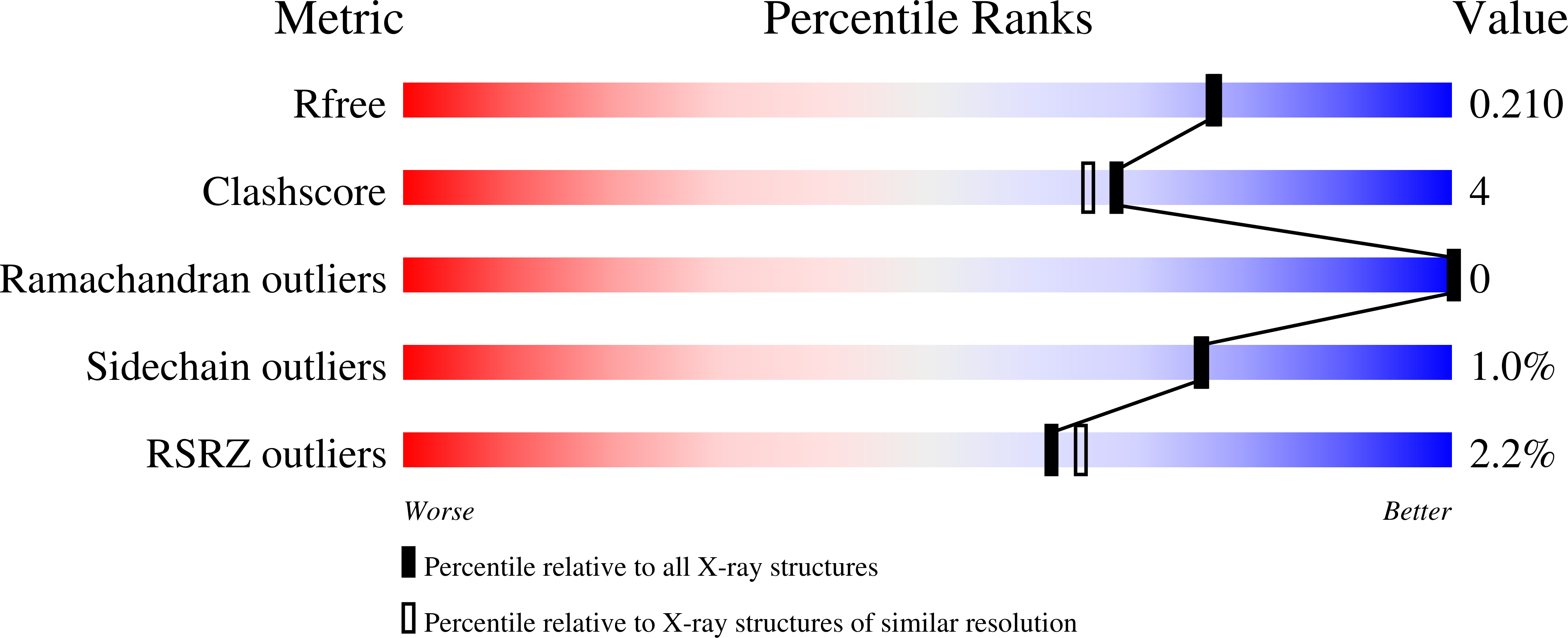
Structural Basis for the Propagation of Homing Endonuclease-Associated Inteins.
Beyer, H.M., Iwai, H.(2022) Front Mol Biosci 9: 855511-855511
- PubMed: 35372505
- DOI: https://doi.org/10.3389/fmolb.2022.855511
- Primary Citation of Related Structures:
7QSS, 7QST, 7QSU - PubMed Abstract:
Inteins catalyze their removal from a host protein through protein splicing. Inteins that contain an additional site-specific endonuclease domain display genetic mobility via a process termed "homing" and thereby act as selfish DNA elements. We elucidated the crystal structures of two archaeal inteins associated with an active or inactive homing endonuclease domain. This analysis illustrated structural diversity in the accessory domains (ACDs) associated with the homing endonuclease domain. To augment homing endonucleases with highly specific DNA cleaving activity using the intein scaffold, we engineered the ACDs and characterized their homing site recognition. Protein engineering of the ACDs in the inteins illuminated a possible strategy for how inteins could avoid their extinction but spread via the acquisition of a diverse accessory domain.
Organizational Affiliation:
Institute of Biotechnology, University of Helsinki, Helsinki, Finland.