A Multispecific Anti-CD40 DARPin Construct Induces Tumor-Selective CD40 Activation and Tumor Regression.
Rigamonti, N., Veitonmaki, N., Domke, C., Barsin, S., Jetzer, S., Abdelmotaleb, O., Bessey, R., Lekishvili, T., Malvezzi, F., Gachechiladze, M., Behe, M., Levitsky, V., Trail, P.A.(2022) Cancer Immunol Res 10: 626-640
- PubMed: 35319751
- DOI: https://doi.org/10.1158/2326-6066.CIR-21-0553
- Primary Citation of Related Structures:
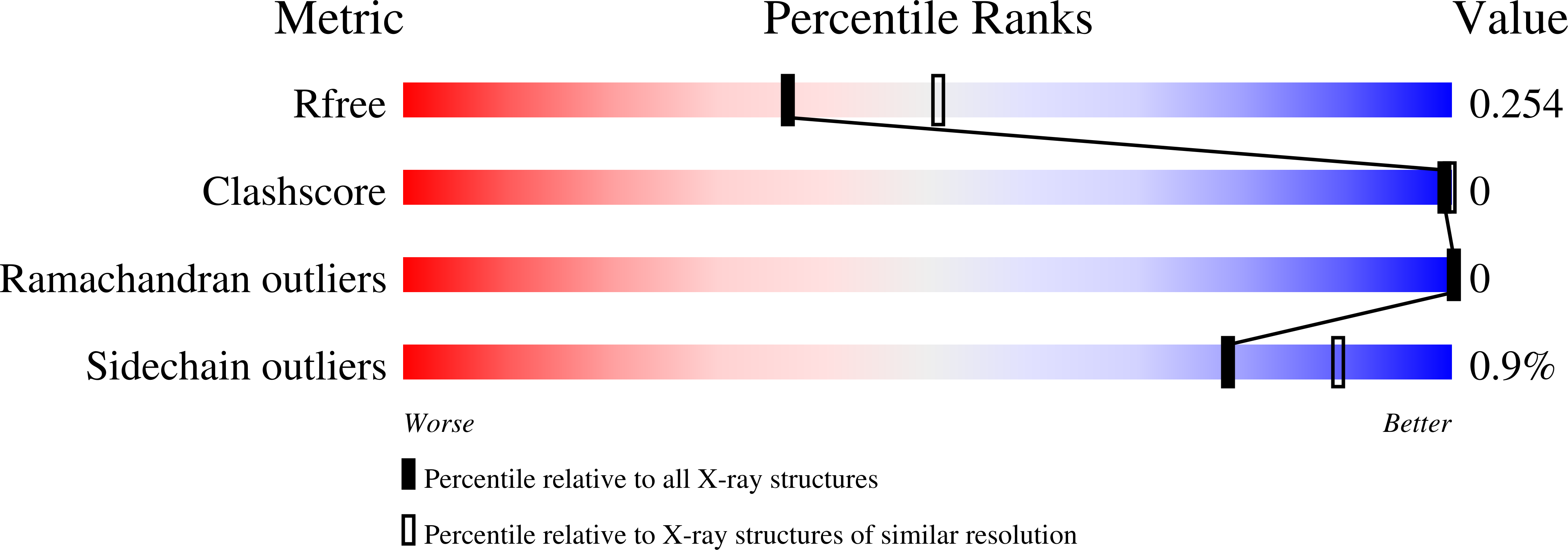
7P3I - PubMed Abstract:
The CD40 receptor is an attractive target for cancer immunotherapy. Although a modest pharmacodynamic effect is seen in patients following administration of CD40-targeting monoclonal antibodies (mAb), the doses that could be safely administered do not result in a meaningful clinical response, most likely due to the limited therapeutic window associated with systemic CD40 activation. To overcome this issue, we developed a multispecific DARPin construct, α-FAPxCD40, which has conditional activity at the site of disease. α-FAPxCD40 activation of CD40 depends on binding to fibroblast activation protein (FAP), a cell-surface protease overexpressed in the stroma of solid tumors. In vitro studies demonstrated that α-FAPxCD40 potently activates human antigen-presenting cells in the presence, but not in the absence, of FAP-positive cells. After intravenous injection, a murine surrogate construct (α-mFAPxCD40) accumulated in FAP-positive tumors, elicited rejection of 88% of these tumors, and induced memory antitumor immunity. Importantly, in contrast to the mouse anti-CD40 tested in parallel, the in vivo antitumor activity of α-mFAPxCD40 was associated neither with elevated blood cytokines nor with hepatotoxicity, both of which contribute to the clinical dose-limiting toxicities of several CD40 mAb. This study demonstrates that α-(m)FAPxCD40 engages CD40 in an FAP-restricted manner, leading to tumor eradication without signs of peripheral toxicity. This distinct preclinical profile suggests that a favorable therapeutic index may be achieved in humans. It further supports the development of α-FAPxCD40, currently tested in a first-in-human clinical study in patients with solid tumors (NCT05098405).
Organizational Affiliation:
Molecular Partners AG, Zurich-Schlieren, Switzerland.