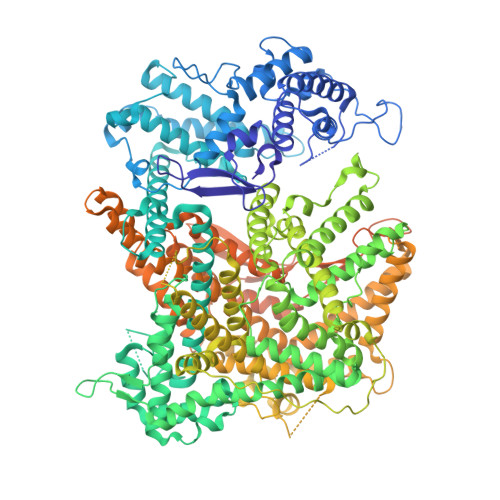
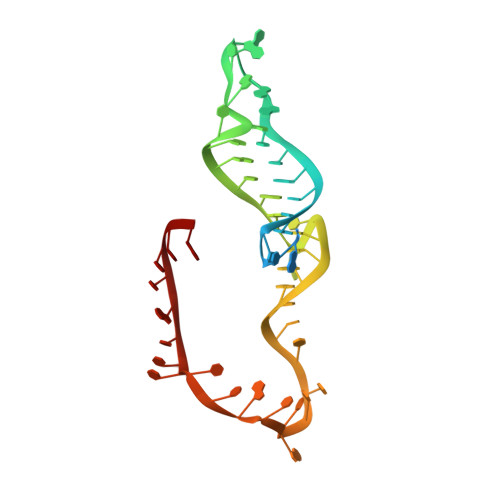
Structure and mechanism of the RNA dependent RNase Cas13a from Rhodobacter capsulatus.
Kick, L.M., von Wrisberg, M.K., Runtsch, L.S., Schneider, S.(2022) Commun Biol 5: 71-71
- PubMed: 35058543
- DOI: https://doi.org/10.1038/s42003-022-03025-4
- Primary Citation of Related Structures:
7OS0 - PubMed Abstract:
Cas13a are single-molecule effectors of the Class II, Type VI family of CRISPR-Cas systems that are part of the bacterial and archaeal defense systems. These RNA-guided and RNA-activated RNA endonucleases are characterized by their ability to cleave target RNAs complementary to the crRNA-spacer sequence, as well as bystander RNAs in a sequence-unspecific manner. Due to cleavage of cellular transcripts they induce dormancy in the host cell and thus protect the bacterial population by aborting the infectious cycle of RNA-phages. Here we report the structural and functional characterization of a Cas13a enzyme from the photo-auxotrophic purple bacteria Rhodobacter capsulatus. The X-ray crystal structure of the RcCas13a-crRNA complex reveals its distinct crRNA recognition mode as well as the enzyme in its contracted, pre-activation conformation. Using site-directed mutagenesis in combination with mass spectrometry, we identified key residues responsible for pre-crRNA processing by RcCas13a in its distinct catalytic site, and elucidated the acid-base mediated cleavage reaction mechanism. In addition, RcCas13a cleaves target-RNA as well as bystander-RNAs in Escherichia coli which requires its catalytic active HEPN (higher eukaryotes and prokaryotes nucleotide binding) domain nuclease activity. Our data provide further insights into the molecular mechanisms and function of this intriguing family of RNA-dependent RNA endonucleases that are already employed as efficient tools for RNA detection and regulation of gene expression.
Organizational Affiliation:
Ludwig-Maximilians University, Department of Chemistry, Institute for Chemical Epigenetics, Würmtalstr. 201, 81375, Munich, Germany.