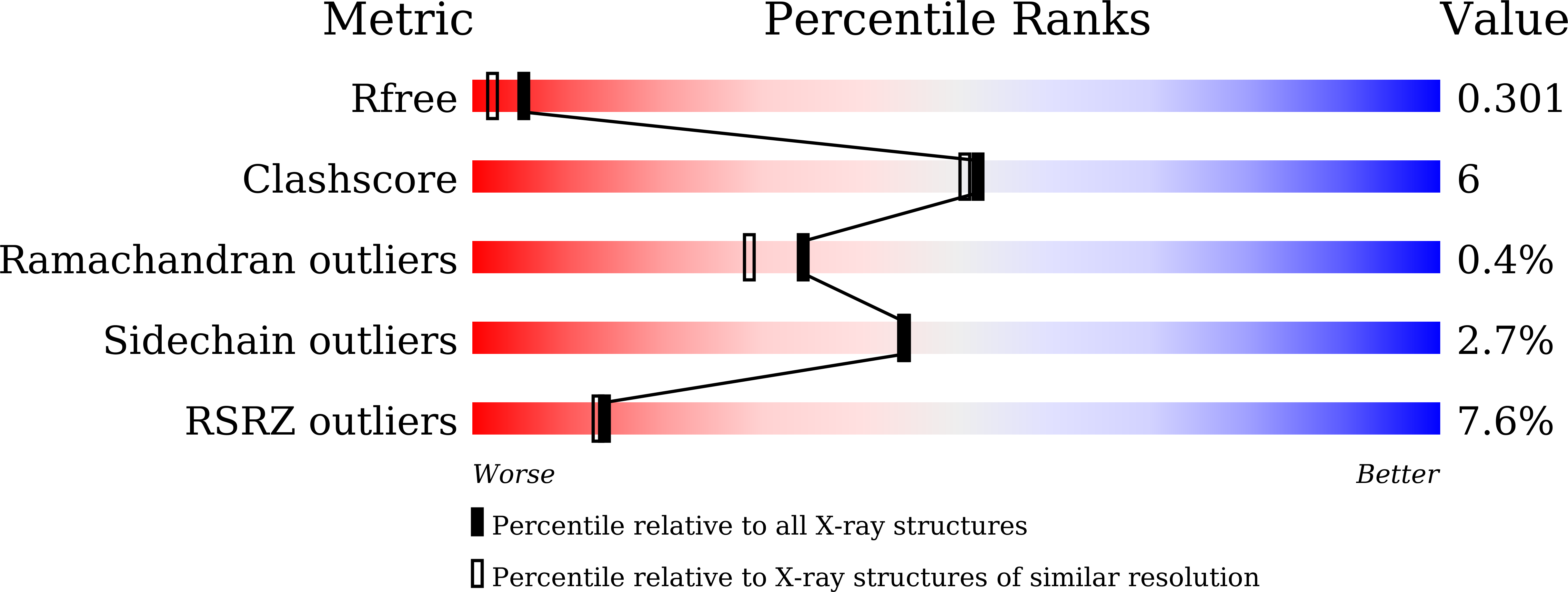
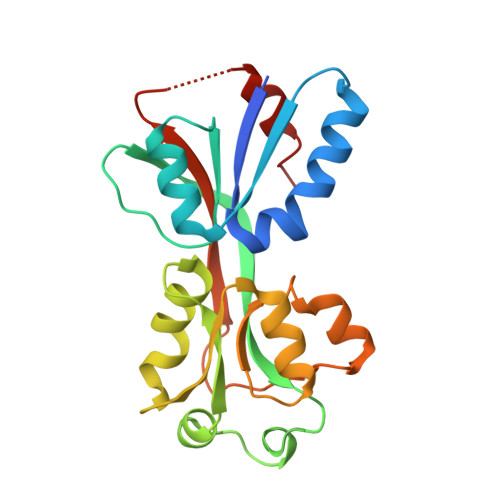
Crystal structures of YeiE from Cronobacter sakazakii and the role of sulfite tolerance in gram-negative bacteria.
Hong, S., Kim, J., Cho, E., Na, S., Yoo, Y.J., Cho, Y.H., Ryu, S., Ha, N.C.(2022) Proc Natl Acad Sci U S A 119: e2118002119-e2118002119
- PubMed: 35271389
- DOI: https://doi.org/10.1073/pnas.2118002119
- Primary Citation of Related Structures:
7ERP, 7ERQ, 7FDF - PubMed Abstract:
SignificanceYeiE has been identified as a master virulence factor of Cronobacter sakazakii . In this study, we determined the crystal structures of the regulatory domain of YeiE in complex with its physiological ligand sulfite ion (SO 3 2- ). The structure provides the basis for the molecular mechanisms for sulfite sensing and the ligand-dependent conformational changes of the regulatory domain. The genes under the control of YeiE in response to sulfite were investigated to reveal the functional roles of YeiE in the sulfite tolerance of the bacteria. We propose the molecular mechanism underlying the ability of gram-negative pathogens to defend against the innate immune response involving sulfite, thus providing a strategy to control the pathogenesis of bacteria.
Organizational Affiliation:
Department of Agricultural Biotechnology, Department of Food and Animal Biotechnology, Seoul National University, Seoul 08826, Republic of Korea.